But if your body is low on these two amino acids, then cysteine supplementation may become necessary. Amino acids are molecules that combine to form proteins. Together, they are considered the building blocks of life that support our cell structure and processes. It’s important to know that the body cannot manufacture NAC nor is it found in foods. Like cysteine, NAC bonds with glutamine and glycine to form glutathione, a powerful antioxidant, and aids in regulating glutamate. The most important N-acetyl cysteine mechanisms of action are its antioxidant and anti-inflammatory effects. FDA-approved, NAC is commonly used in treating the side effects of acetaminophen poisoning. Taken in large doses, acetaminophen can cause damage to the liver. As a powerful antioxidant, NAC can lower both oxidative stress and inflammation. Now, numerous studies are pointing to the potential benefits of using NAC to combat the negative impacts of certain chemical imbalances that can occur in our system and lead to the development of some neurological disorders. So, why is NAC something you should know more about? Keep reading for details about the research and how this amino acid may play a critical role in brain health! NAC for neurodegenerative disease. There are many reasons why NAC can be helpful in neurodegenerative disease. In addition to being a tool to fight oxidative stress and inflammation, NAC easily crosses the blood-brain barrier (BBB), promotes neurogenesis, has neuroprotective properties, regulates glutamate which is an excitatory neurotransmitter, and increases glutathione levels in the body and brain. Note: Glutamate plays a major role in learning and memory. For your brain to function properly, glutamate needs to be present in the right concentration in the right places at the right time. Too much glutamate is associated with diagnoses such as Parkinson's disease, Alzheimer's disease (AD), multiple sclerosis (MS), amyotrophic lateral sclerosis (ALS), and Huntington's disease. Understanding the role of oxidative stress in neurodegenerative disease. Oxidative stress can occur when there is an imbalance of free radicals and antioxidants in the body. We can think of it much like the accumulation of rust on a car. With years of wear and tear, rust can slowly begin to build unnoticed. But eventually, rust and its subsequent damage can overtake the entire structure. In this same way, unabated oxidative stress can impact our overall health and our brain’s wellbeing. During normal metabolic processes, the body’s cells produce free radicals, which are very unstable molecules; cells also produce antioxidants that neutralize these free radicals. In general, the body can maintain a balance between antioxidants and free radicals. However, free radicals can build up in cells and cause damage to other molecules such as DNA, lipids, and proteins, etc., which can lead to aging and chronic disease including neurodegenerative disease and cancer. What are the risk factors for oxidative stress? Factors that may increase a person’s risk of long-term oxidative stress include:
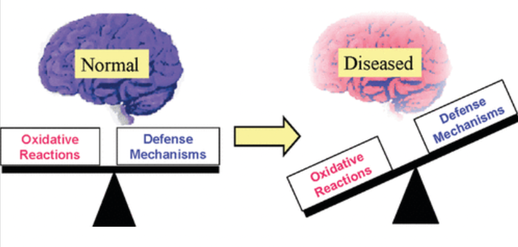
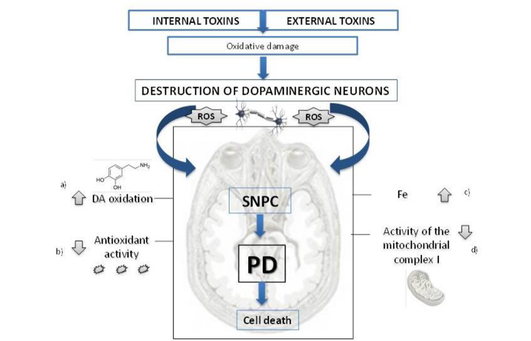
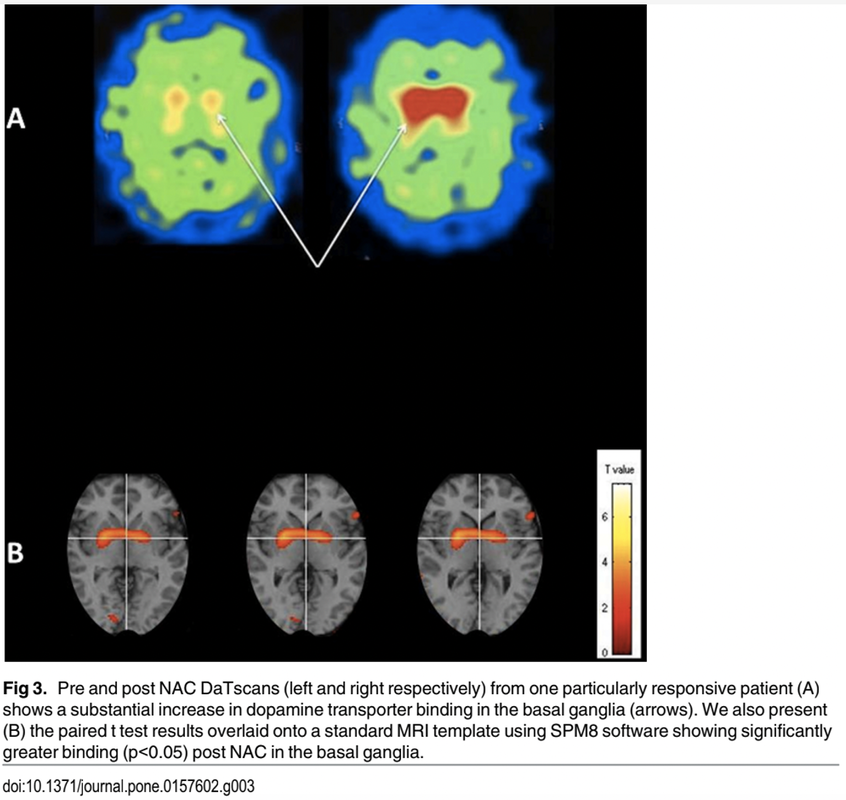
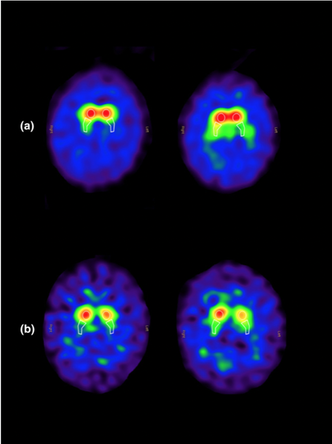
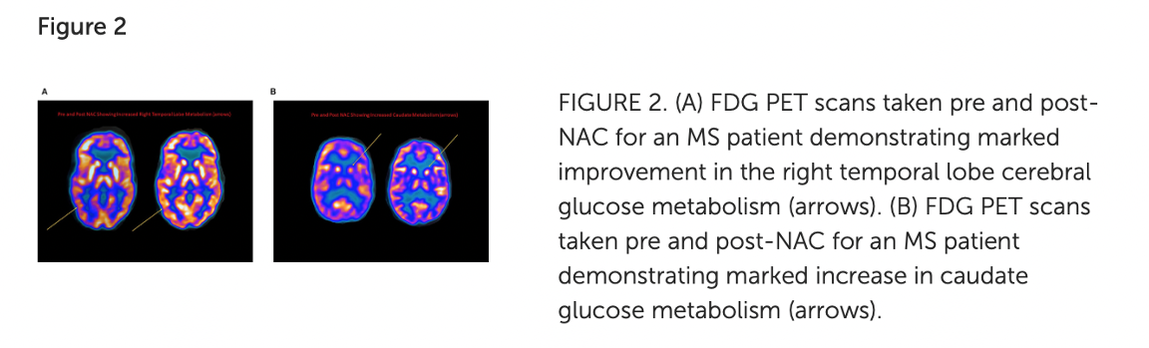
Oxidative stress and the brain. Because its cells require a substantial amount of oxygen, our brain is particularly vulnerable to the effects of oxidative stress. In fact, according to a 2018 review, the brain alone uses 20 percent of the total amount of oxygen the body needs to fuel itself and all of its processes. Brain cells utilize oxygen to perform intense metabolic activities that subsequently generate free radicals. These free radicals help support brain cell growth, neuroplasticity, and cognitive functioning. But in the presence of oxidative stress, excess free radicals can damage structures inside brain cells and even cause cell death, which may increase the risk of neurodegenerative disease. Oxidative stress also alters essential proteins, such as amyloid-beta peptides, and may modify these peptides in way that contributes to the accumulation of amyloid plaques in the brain. It also reduces central nervous system (CNS) levels of glutathione, the major free radical scavenger in the brain and this sets up a dangerous situation for the CNS - remember the analogy of a rusty car we mentioned earlier? We know that the CNS is especially vulnerable to oxidative insult due to the high rate of 02 utilization – and the result is neurotoxicity. Image credit: https://pubs.acs.org/doi/10.1021/tx700210 Glutathione regulates aging and neurodegeneration. Glutathione is an antioxidant found naturally occurring in the body. Also known as GSH, it is produced by the liver and nerve cells in the CNS and is made up of three amino acids: glycine, L-cysteine, and L-glutamate. Glutathione can help metabolize toxins, break down free radicals, support immune function, and more. In the brain, glutathione plays a critical role in the antioxidant defense system and the maintenance of redox homeostasis in neurons. Additionally, it serves many pivotal functions in the CNS including the modulation of cellular differentiation and proliferation, apoptosis, enzyme activation, metal transport in cells, and neurotransmission. GSH is also integrally involved in neuronal defense against damage from reactive oxidative species (ROS) associated with aging. ROS is a type of unstable molecule that contains oxygen and that easily reacts with other molecules in a cell. Cellular redox homeostasis is an essential and dynamic process that ensures the balance between reducing and oxidizing reactions within cells and regulates a plethora of biological responses and events. But as we age, our brain levels of glutathione decrease – and studies have identified GSH depletion in the brain to be commonly found in patients with neurodegenerative diseases such as Alzheimer’s, MS, and Parkinson’s. Scientific evidence confirms that a low level of glutathione can even impair short-term and long-term mechanisms of synaptic plasticity, the process by which our brain adjusts how neurons interact and process information. Boosting glutathione levels in the brain. It’s important to note that there are ongoing differences of opinion as to whether GSH given orally or intravenously can cross the BBB. According to this source, GSH can “hardly cross the BBB, so that brain concentrations cannot be increased by peripheral administration of GSH.” On the other hand, NAC is readily taken up by neurons and it can help to boost neuronal glutathione levels as it can cross the BBB. And it has been shown to be truly safe and effective, even at relatively high doses. Oxidative stress in Parkinson’s - can NAC help? Parkinson's disease is caused by a loss of nerve cells or neurons in part of the brain called the substantia nigra, located just above the brainstem. Symptoms of the disorder may include muscular rigidity, bradykinesia, tremors in resting limbs, and problems with postural balance. Loss of neurons can result in reduction of dopamine, a vital neurotransmitter responsible for the body’s smooth, controlled movements as well as our motivation and reward mechanism. The importance of oxidative stress in Parkinson’s disease (PD) cannot be overstated and evidence suggests that reactive oxygen species (ROS) are derived from dopamine itself. One of the reasons for the high levels of oxidative stress is because glutathione, an important antioxidant in the neurons, has been found to be depleted in the brain of PD patients and the magnitude of glutathione depletion appears to parallel the severity of the disease. Studies carried out on cultured human cortical neurons have highlighted that NAC may protect against the programmed cell death (PCD) induced by dopamine and in synaptic mitochondrial preparations in aged mice. If cells are no longer needed, they essentially self-destruct by activating an intracellular death program; in Parkinson’s disease, dopamine neurons in the substantia nigra of human brain are selectively vulnerable. Mitochondrial dysfunction plays a major role in the pathogenesis of PD, but studies have found NAC can also reduce this negative action by inhibiting oxidative stress. Different pathways contribute to the substantia nigra pars compacta (SNPC) neurons vulnerability to oxidative damage including (a) high susceptibility of DA auto-oxidation, (b) reduced antioxidant activity such as glutathione, and (c) increased iron concentration d) deficits in mitochondrial complex I of the respiratory chain. Image Credit: https://www.intechopen.com/chapters/51482 This study in 2016, demonstrated for the first time a potential direct effect of NAC on the dopamine system in PD patients. Patients were treated with IV NAC with a dose of 50mg/kg mixed into 200ml of D5W infused over approximately one-hour 1x per week. Subjects took the 600mg NAC tablets 2x per day on the days that they did not receive the IV NAC. The control group continued standard of care medication. The treatment continued for 90 days and then a DaTscan was taken and as well a repeat UPDRS scale (Unified Parkinson's Disease Rating Scale (UPDRS) is a rating tool used to gauge the severity and progression of Parkinson's disease in patients) The post treatment DaTscan showed a ‘substantial increase in dopamine transporter binding in the basal ganglia. Dopamine transporter is a protein that works to recycle dopamine after its release in the brain. In Parkinson’s, dopamine transporter levels may be reduced to up to 70%. But this study showed that NAC increased this protein, thereby making more dopamine available. The UPDRS score in the NAC treated group was improved from baseline. Diagram Credit: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0157602 In a 2019 investigation, forty-two patients with PD were randomized to either weekly intravenous infusions of NAC (50 mg/kg) plus oral doses (500 mg twice per day) for 3 months or standard of care only. Participants received pre- and post-NAC administration brain imaging studies using ioflupane (DaTscan) to measure dopamine transporter (DAT) binding. In the NAC group, significantly increased DAT binding was found in the caudate and putamen compared with controls along with significantly improved PD symptoms as seen in this diagram. In these images, the ones on the right are post NAC. The results suggest once again that NAC may positively affect the dopaminergic system in patients with PD, with corresponding positive clinical effects. What about NAC in MS? Scientific evidence has found that oxidative stress plays a major role in the pathogenesis of multiple sclerosis (MS). The results of this study suggest that NAC administration alters resting CBF (cerebral blood flow) in MS patients, and this is associated with qualitative improvements in cognition and attention. The experimental group received NAC intravenously (50 mg/kg) once per week and orally (500mg 2x/day) the other six days. The study lasted 2 months. Image credit: https://pubmed.ncbi.nlm.nih.gov/32117038/ Additional efforts focused on MS research relevant to the use of NAC include:
The dose given was 50 mg/kg mixed into 200 ml of D5W infused over ~1 h 1x per week. Subjects additionally took 500 mg NAC tablets twice daily on the days that they did not receive the intravenous form of NAC. The study continued X 2 months.
It is important to remember that the body requires both free radicals AND antioxidants – an imbalance that results in too many or too few of either may lead to health problems. KEY TAKEAWAYS:
Optimizing our neurological wellbeing. If you’re a regular reader of our blogs, you know that we often emphasize the importance of following a healthy lifestyle as the first important step in promoting brain health. As this blog has noted, reducing oxidative stress is key to also reducing harmful inflammation and the resulting problems that can arise from it. And as we’ve also frequently discussed, there is a direct link between the state of our gut and the health of our brain! Dr. Terry Wahls, who following her MS diagnosis from over 20 years ago designed and adhered to a unique protocol that includes dietary controls, recently wrote about the benefits of intermittent fasting and caloric restriction as a tool for disease management. Our recommendations always start with choosing a healthy nutritional approach based on whole foods over processed, adequate proteins, plenty of vegetables and some low sugar fruits, complex carbohydrates over refined products, and minimal sugary treats. Always opt for healthy fats like olive oil, the heart of the Mediterranean diet. It is especially important to choose, when possible, non-GMO foods and sustainably raised produce and meat. Inset: You don’t have to totally deprive yourself of something sweet! If you too are a chocolate lover, there are ways to satisfy that craving in moderation that also could benefit your brain: https://www.suzannegazdamd.com/blog/listen-to-your-brain-and-go-ahead-and-eat-the-chocolate We are what – and when – we eat. We’ve recently discussed in another blog how the state of our gut health and microbiome could play a big role in the prognosis of autoimmune neurological disease including multiple sclerosis. As such, we often recommend intermittent fasting to optimize brain health. In this trial, just one of many investigations, MS patients that used intermittent caloric restriction over a period of 12 weeks showed therapeutic effects with a marked increase in brain volume measurement as determined by MRIs, as well as improved blood flow and reduction in neuroinflammation. Restricting the hours when you eat has been shown as well to significantly improve memory, according to a study in the Journal of the Academy of Nutrition and Dietetics. In this study, after 4 weeks of intermittent fasting, performance on a spatial planning and working memory task and on a working memory capacity test increased significantly. Additional research on animals has found that intermittent fasting improves learning and memory. As we continually emphasize, it’s best to stick to an anti-inflammatory diet that includes a variety of healthy, whole foods. Additionally, we should:
High levels of oxidative stress are implicated in aging and neurodegenerative disease. But utilizing proven measures to address factors that can fuel excess oxidative stress and employing antioxidant therapies may offer significant health benefits. Indeed, in restoring cellular glutathione, a key antioxidant that decreases with age and in neurodegenerative disease, it’s possible that NAC may be an especially important option. It really is so important that each of us commits to being an informed and active participant in our own health goals! As part of this process, it’s crucial to learn as much as we can about the ways that food and other lifestyle influences can affect our wellbeing. By identifying and better understanding the many resources we all have available, we can ideally feel our best and more fully enjoy our lives both today and tomorrow. That is our hope and our commitment to every patient, so please let us know how we can help! In health and healing, Dr. Suzanne Gazda Additional reflections…what about NAC in cases of long COVID and the vaccine injured? We also recommend NAC in our long COVID and post-vaccine recovery protocols. Not only can NAC reduce oxidative stress and inflammation, but it has antiplatelet effects which can benefit the microclotting, endothelial activation, and platelet activation going on as a result of lingering spike protein. Studies also have shown that NAC may be a useful tool to degrade spike protein. In long COVID, spike protein not only causes direct damage to the mitochondria, but it also sets in motion a redox shift catalyzing high levels of oxidative stress and cellular compromise. This unrelenting cycle drives inflammation, chronic fatigue, brain dysfunction, accelerated aging and much more. Reactive oxidative species or ROS can trigger inflammation, damage the endothelium, lead to microthrombi and neuroinflammation, while promoting the formation of autoantibodies and disrupting neurotransmitter assembly. This unrelenting anabolism, the biochemical process in metabolism where the simple molecules combine to generate complex molecules, can lead to the formation of cytokine storms, chronic fatigue, chronic inflammation, or neurodegenerative diseases. For more about long COVID, please see our extensive series at: https://www.suzannegazdamd.com/blog---long-covid References and additional reading: Lauterburg BH, Corcoran GB, Mitchell JR. Mechanism of action of N-acetylcysteine in the protection against the hepatotoxicity of acetaminophen in rats in vivo. J Clin Invest. 1983;71(4):980-991. doi:10.1172/jci110853. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC436956/ Zhou Y, Danbolt NC. Glutamate as a neurotransmitter in the healthy brain. J Neural Transm (Vienna). 2014;121(8):799-817. doi:10.1007/s00702-014-1180-8 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4133642/ Olsen, N. How can antioxidants benefit our health? Medical News Today. (2018) https://www.medicalnewstoday.com/articles/301506.php Sampson, S. How does oxidative stress affect the body? Medical News Today. (2019) https://www.medicalnewstoday.com/articles/324863 Barhoumi, T. et al. SARS-CoV-2 Coronavirus Spike Protein-Induced Apoptosis, Inflammatory, and Oxidative Stress Responses in THP-1-Like-Macrophages: Potential Role of Angiotensin-Converting Enzyme Inhibitor (Perindopril). (2021) https://www.frontiersin.org/articles/10.3389/fimmu.2021.728896/full?trk=public_post_comment-text Sun, Q., Li, L., Jin, F. et al. SARS-CoV-2 Spike Protein S1 Exposure Increases Susceptibility to Angiotensin II-Induced Hypertension in Rats by Promoting Central Neuroinflammation and Oxidative Stress. Neurochem Res (2023). https://doi.org/10.1007/s11064-023-03949-1 https://link.springer.com/article/10.1007/s11064-023-03949-1 Sampson, S. How much should I weigh for my height and age? Medical News Today. (2023) https://www.medicalnewstoday.com/info/obesity/how-much-should-i-weigh.php Cobley JN, Fiorello ML, Bailey DM. 13 reasons why the brain is susceptible to oxidative stress. Redox Biol. 2018;15:490-503. doi:10.1016/j.redox.2018.01.008 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5881419/ Cheignon C, Tomas M, Bonnefont-Rousselot D, Faller P, Hureau C, Collin F. Oxidative stress and the amyloid beta peptide in Alzheimer's disease. Redox Biol. 2018;14:450-464. doi:10.1016/j.redox.2017.10.014 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5881419/ Gazda, S. Alpha synuclein in neurodegenerative disease. suzannegazdamd.com (2020) https://www.suzannegazdamd.com/scientifically-speaking1/alpha-synuclein-in-neurodegenerative-disease Wu G, Fang YZ, Yang S, Lupton JR, Turner ND. Glutathione metabolism and its implications for health. J Nutr. 2004. https://www.sciencedirect.com/science/article/pii/S0022316623026639?via%3Dihub Aoyama K. Glutathione in the Brain. Int J Mol Sci. 2021;22(9):5010. Published 2021 May 9. doi:10.3390/ijms22095010. Le Gal K, Schmidt EE, Sayin VI. Cellular Redox Homeostasis. Antioxidants (Basel). 2021;10(9):1377. Published 2021 Aug 28. doi:10.3390/antiox10091377 Hara, Y. et al. Evaluation of the neuroprotective potential of N-acetylcysteine for prevention and treatment of cognitive aging and dementia. J Prev Alz Dis. (2017). https://www.jpreventionalzheimer.com/all-issues.html?article=322 Farr SA, Poon HF, Dogrukol-Ak D et al. The antioxidants alpha-lipoic acid and N-acetylcysteine reverse memory impairment and brain oxidative stress in aged SAMP8 mice. J Neurochem. 2003. https://pubmed.ncbi.nlm.nih.gov/12603840/ Katz M, Won SJ, Park Y, et al. Cerebrospinal fluid concentrations of N-acetylcysteine after oral administration in Parkinson's disease. Parkinsonism Relat Disord. 2015;21(5):500-503. doi:10.1016/j.parkreldis.2015.02.020. https://pubmed.ncbi.nlm.nih.gov/25765302/ Wang HL, Zhang J, Li YP, Dong L, Chen YZ. Potential use of glutathione as a treatment for Parkinson's disease. Exp Ther Med. 2021;21(2):125. doi:10.3892/etm.2020.9557 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7751460/ Bjørklund G, Peana M, Maes M, Dadar M, Severin B. The glutathione system in Parkinson's disease and its progression. Neurosci Biobehav Rev. 2021;120:470-478. doi:10.1016/j.neubiorev.2020.10.004. https://pubmed.ncbi.nlm.nih.gov/33068556/ Moon HE, Paek SH. Mitochondrial Dysfunction in Parkinson's Disease. Exp Neurobiol. 2015;24(2):103-116. doi:10.5607/en.2015.24.2.103. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4479806/ Martínez Banaclocha M. N-acetylcysteine elicited increase in complex I activity in synaptic mitochondria from aged mice: implications for treatment of Parkinson's disease. Brain Res. 2000;859(1):173-175. doi:10.1016/s0006-8993(00)02005-9. Winston Tse-Hou Kwok, Haejin Angela Kwak, Ana Cristina Andreazza. N-acetylcysteine modulates rotenone-induced mitochondrial Complex I dysfunction in THP-1 cells. Mitochondrion. (2023). ISSN 1567-7249. https://doi.org/10.1016/j.mito.2023.07.001. Monti, D. et al. N-Acetyl Cysteine May Support Dopamine Neurons in Parkinson's Disease: Preliminary Clinical and Cell Line Data. PLOS One. (2016). https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0157602 Monti, D.A., Zabrecky, G., Kremens, D., Liang, T.-W., Wintering, N.A., Bazzan, A.J., Zhong, L., Bowens, B.K., Chervoneva, I., Intenzo, C. and Newberg, A.B. (2019), N-Acetyl Cysteine Is Associated With Dopaminergic Improvement in Parkinson's Disease. Clin. Pharmacol. Ther., 106: 884-890. https://doi.org/10.1002/cpt.1548.https://ascpt.onlinelibrary.wiley.com/doi/10.1002/cpt.1548 Ohl K, Tenbrock K, Kipp M. Oxidative stress in multiple sclerosis: Central and peripheral mode of action. Exp Neurol. 2016;277:58-67. doi:10.1016/j.expneurol.2015.11.010 Shahrampour S, Heholt J, Wang A, et al. N-acetyl cysteine administration affects cerebral blood flow as measured by arterial spin labeling MRI in patients with multiple sclerosis. Heliyon. 2021;7(7):e07615. Published 2021 Jul 16. doi:10.1016/j.heliyon.2021.e07615 Schoeps, V.A., et al. N-Acetyl Cysteine as a Neuroprotective Agent in Progressive Multiple Sclerosis (NACPMS) trial: Study protocol for a randomized, double-blind, placebo-controlled add-on phase 2 trial. Contemporary Clinical Trials. Volume 122, 2022, 106941, ISSN 1551-7144. https://doi.org/10.1016/j.cct.2022.106941. https://www.sciencedirect.com/science/article/pii/S1551714422002671 Majdinasab, N. et al. The comparison of efficacy and safety of N-acetylcysteine and amantadine on fatigue, degree of disability, and quality of life of patients with MS that referred to the MS Association of Khuzestan Province. NeuroQuantology. (2022). Volume 20, Issue 16. Page 3432-3441| doi: 10.48047/NQ.2022.20.16.NQ880349. Khalatbari Mohseni, G, Hosseini, SA, Majdinasab, N, Cheraghian, B. Effects of N-acetylcysteine on oxidative stress biomarkers, depression, and anxiety symptoms in patients with multiple sclerosis. Neuropsychopharmacol Rep. 2023; 00: 1– 9. https://doi.org/10.1002/npr2.12360 Marseglia L, Manti S, D'Angelo G, et al. Oxidative stress in obesity: a critical component in human diseases. Int J Mol Sci. 2014;16(1):378-400. Published 2014 Dec 26. doi:10.3390/ijms16010378 Rahmani F, Ghezzi L, Tosti V, et al. Twelve Weeks of Intermittent Caloric Restriction Diet Mitigates Neuroinflammation in Midlife Individuals with Multiple Sclerosis: A Pilot Study with Implications for Prevention of Alzheimer's Disease. J Alzheimers Dis. 2023;93(1):263-273. doi:10.3233/JAD-221007 Wang, Z. et al. Chronic Intermittent Fasting Improves Cognitive Functions and Brain Structures in Mice. PLOS One. (2013). https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0066069#s1 Farooq A, Herrera CP, Almudahka F, Mansour R. A Prospective Study of the Physiological and Neurobehavioral Effects of Ramadan Fasting in Preteen and Teenage Boys. J Acad Nutr Diet. 2015;115(6):889-897. doi:10.1016/j.jand.2015.02.012 Gibson, K.R., Winterburn, T.J., Barrett, F. et al. Therapeutic potential of N-acetylcysteine as an antiplatelet agent in patients with type-2 diabetes. Cardiovasc Diabetol 10, 43 (2011). https://doi.org/10.1186/1475-2840-10-43 Debnath U, Mitra A, Dewaker V, Prabhakar YS, Tadala R, Krishnan K, et al. N-acetyl cysteine: A tool to perturb SARS-CoV-2 spike protein conformation. ChemRxiv. Cambridge: Cambridge Open Engage. 2021. [preprint] Amen Clinics. Memory and dementia. https://www.amenclinics.com/conditions/memory-problems-and-dementia/ Clough E, Inigo J, Chandra D, et al. Mitochondrial Dynamics in SARS-COV2 Spike Protein Treated Human Microglia: Implications for Neuro-COVID [published correction appears in J Neuroimmune Pharmacol. 2021 Dec 11;:]. J Neuroimmune Pharmacol. 2021;16(4):770-784. doi:10.1007/s11481-021-10015-6. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8487226/ Gazda, S. Antioxidant therapy with NAC for MS. suzannegazdamd.com. (2021) https://www.suzannegazdamd.com/blog---ms-in-the-news/antioxidant-therapy-with-nac-for-ms Gazda, S. What does gut health have to do with MS? suzannegazdamd.com. (2023) https://www.suzannegazdamd.com/blog---ms-in-the-news/what-does-gut-health-have-to-do-with-ms-research-shows-it-could-be-significant Douglass, H. Increasing Glutathione Levels Lowers Alzheimer’s Pathology and Improves Cognitive Decline. UNSW – Centre for Healthy Brain Ageing. (2021). https://cheba.unsw.edu.au/news/increasing-glutathione-levels-lowers-alzheimers-pathology-and-improves-cognitive-decline-0
0 Comments
Your comment will be posted after it is approved.
Leave a Reply. |
AuthorDr. Suzanne Gazda, Integrative Neurology Archives
February 2024
Categories |

 RSS Feed
RSS Feed