What is the gut microbiome? Our gut is home to a very large number of microbes collectively known as the gut microbiota, comprising more than 38 trillion microbes, which is at least as many cells as we have in our whole body! Their collective genome, known as the gut microbiome, contains 150 times more genes than the human genome. Many studies offer strong evidence for the role of the commensal gut microbiota in brain function and behavior. Commensal refers to a type of relationship between two different organisms that “eat from the same dish”. In this kind of relationship, neither organism benefits from the other or provokes any harm; essentially these organisms coexist peacefully in a neutral relationship – which tells us something about why maintaining the balance of this relationship is so critical to health Many potential pathways are involved in the gut-brain axis, which is the bidirectional communication between the gut microbiota and the brain, such as:
The gut-brain axis between the gastrointestinal system and the central nervous system (CNS) is critical to homeostasis (the self-regulating process by which our system maintains stability) between neural (both enteric and central nervous systems), hormonal and immunological signaling. We’ve found that dysbiosis, the imbalance of bacteria in our digestive system, of gut microbial function has been associated with behavioral and neurophysical deficits; therefore, research focused on developing new therapeutic strategies to treat neurodegenerative and neuropsychiatric disorders starts with first targeting the gut microbiota. However, as we’ve often noted in our body of writing, the Western diet, which is characterized among other things by red meat, processed foods, sugar, dairy and refined grains together with sedentary lifestyles, results in modifications to the gut microbiota. This change is thought to contribute in part to increased incidences of chronic inflammatory disorders, such as cardiovascular disease (CVD), obesity, depression, allergies, diabetes and autoimmune disorders.1 It would appear that Hippocrates, the Greek physician who is considered to be the “father of medicine”, was indeed on to something when he said “all disease begins in the gut”! It’s fascinating to think that this wisdom from so long ago still holds so much truth in the foundations of good health today. 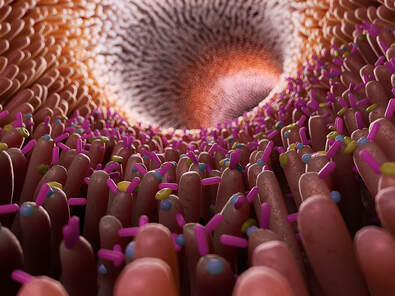 The Gut Microbiome: An Ecosystem of Wonder. Given that the gut microbiota has been identified as a critical regulator of immune responses, we now know too that it appears to play an important role in multiple sclerosis (MS) and about which we had previously written as well. Recent findings from the global collaborative International Multiple Sclerosis Microbiome Study (iMSMS) identified “modest, but statistically significant MS-linked changes in gut microbiota composition and function in patients with the disease.” This was according to study findings presented in September 2020 at the 8th Joint American Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) and European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) MSVirtual2020 event. The iMSMS is the largest reported study of the gut microbiome in patients with MS. The goal of this study was to identify microbes, genes, and pathways involved in MS pathogenesis, as well as to examine the effect of treatment on microbiome changes. This analysis of the iMSMS included a total of 576 patients with MS and household healthy control pairs who were enrolled from 7 centers in the United States, Europe, and South America. Study researchers collected stool samples and evaluated these with both 16S and shallow whole metagenome shotgun sequencing. Patterns of variation of the gut microbiome were analyzed using univariate and multivariate linear regression analyses. There was a significant difference between patients with MS and healthy controls in terms of beta diversity. The microbiomes of untreated patients with MS were significantly enriched for multiple Akkermansia species, including the mucin-degrading bacterium Akkermansia muciniphila In cases with MS, the most significantly enriched bacteria included Ruminococcus torques and Eisenbergiella tayi. In the untreated patients with MS, Faecalibacterium prausnitzii, a main butyrate producer, was significantly reduced. Additionally, untreated patients with MS exhibited slightly increased functional pathways of L-tryptophan biosynthesis and L-threonine biosynthesis. Patients with MS who did receive treatment had increased 5-aminoimidazole ribonucleotide biosynthesis Further research in this arena will certainly help guide us with targeted regimens to improve the health of the compromised microbiome in MS. A healthy gut begins with a healthy diet…more to come on the specifics of diet and nutrition in disease prevention and you can find several articles to date in our blog library as well. In health, Dr. Suzanne Gazda References and additional reading: 1 Oriach, C.S., Robertson, R.C., Stanton, C., Cryan, J.F., Dinan, T.G. Food for thought: The role of nutrition in the microbiota-gut–brain axis. Clinical Nutrition Experimental. Volume 6, April 2016, Pages 25-38 https://www.sciencedirect.com/science/article/pii/S235293931600004X#bib16 Brody, H. The Gut Microbiome. Nature. (29 January 2020) https://www.nature.com/articles/d41586-020-00194-2 Johns Hopkins Medicine https://www.hopkinsmedicine.org/research/advancements-in-research/fundamentals/in-depth/the-gut-where-bacteria-and-immune-system-meet
0 Comments
Your comment will be posted after it is approved.
Leave a Reply. |
AuthorDr. Suzanne Gazda, Integrative Neurology Archives
February 2024
Categories |

 RSS Feed
RSS Feed