Immune Dysregulation -Cytokine storm and uncontrolled inflammation Some of the early clues found were unusually high levels of cytokines, which are the chemical messengers used by the immune system to communicate. In November 2020, Dr. Athena Akrami, a neuroscientist and group leader at University College-London, Sainsbury Wellcome Centre, co-authored a preprint proposing that misfired cytokines, which would lead to a hyperactive immune system, could explain most of the symptoms of long COVID. -Rogue antibodies Scientists have also found an astonishingly high level of proteins produced by the immune system known as autoantibodies, which attack cells and tissues in the body instead of the virus. Many of the autoantibodies were directed against the immune system itself and those autoantibodies were also directed at many other parts of the body, from the brain and central nervous system to blood vessels and platelets. So far, it appears these autoantibodies can linger in some patients who had COVID 19 for many months, if not longer. The science is showing that SARS-CoV-2 might cause the body to generate autoantibodies that attack its own tissues. Some of the infected individuals had autoantibodies against proteins in their blood vessels, heart and brain. Essentially the immune system starts attacking the body and brain. This can result in any number of autoimmune diseases or worsening of underlying autoimmune pathophysiology. SARS-CoV-2 has been shown to drive cross-reactive antibody responses; for example, in one study, researchers Kreye et al. (2020) identified high-affinity SARS-CoV-2-neutralizing antibodies that cross-reacted with gut, kidney, lung, heart, and brain mammalian self-antigens. Antibody-binding in the brain occurred in the basal ganglia, hippocampal formation, olfactory bulb, and cerebral cortex. Another study (Marino Gammazza et al., 2021) found that SARS-CoV-2 proteins can share homology with neuron protein epitopes found within vagus or brainstem nuclei such as the jugular ganglion, nodose ganglion, dorsal motor nucleus, and nucleus ambiguous. Molecular mimicry Molecular mimicry is one of the leading mechanisms by which infectious or chemical agents may induce autoimmunity. It occurs when similarities between foreign and self-peptides favor an activation of autoreactive T or B cells by a foreign-derived antigen in a susceptible individual. In a recent study in the Journal of Immunology, evidence shows that SARS-CoV-2 may cause the body’s immune system to mistakenly attack its own cells and tissues (in the heart, in the brain, the lungs and elsewhere), due to the similarities between the amino acid sequences of coronavirus proteins and those of human proteins. In this mechanism, antibodies formed against SARS-CoV-2, would also bind to human tissue proteins leading to autoimmune reactivity. The investigation identified significant cross-reactivity between SARS-CoV-2 proteins (spike protein, nucleoprotein, envelope protein, and membrane protein) with 55 different human tissues using monoclonal and polyclonal antibodies and performed epitope mapping to identify the relationships. Molecular mimicry may explain multi-organ damage in COVID and in Long Haulers and vaccine injured and suspected of contributing to neuropsychiatric symptoms in severe COVID-19 cases due to molecular mimicry of the N-methyl-D-aspartate (NMDA) receptor. NMDA hypofunction within the brain is associated with memory and learning impairments as well as psychosis, and ultimately with excitotoxicity-related brain injury. Excitotoxicity describes the toxic actions of excitatory neurotransmitters, primarily glutamate; prolonged activation of glutamate receptors start a cascade of neurotoxicity that leads to the loss of neuronal function and cell death.
Reduced interferon production subsequently delays the release of cells that should eliminate detrimental elements and stagnates T-cell response. A recent study, “the BNT162b2 mRNA vaccine against SARS-CoV-2 reprograms both adaptive and innate immune responses,” while not yet peer reviewed, showed that the vaccines altered the production of inflammatory cytokines by innate immune cells following stimulation with both specific (SARS-CoV-2) and non-specific (viral, fungal and bacterial) stimuli.
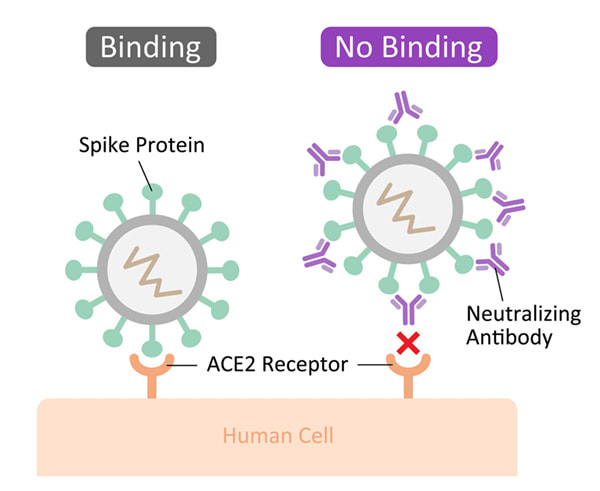
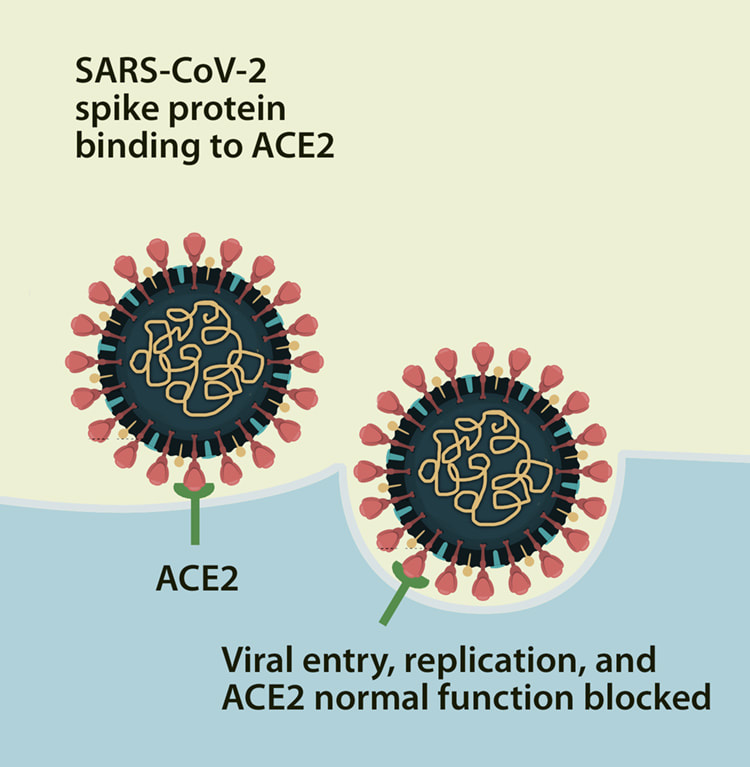
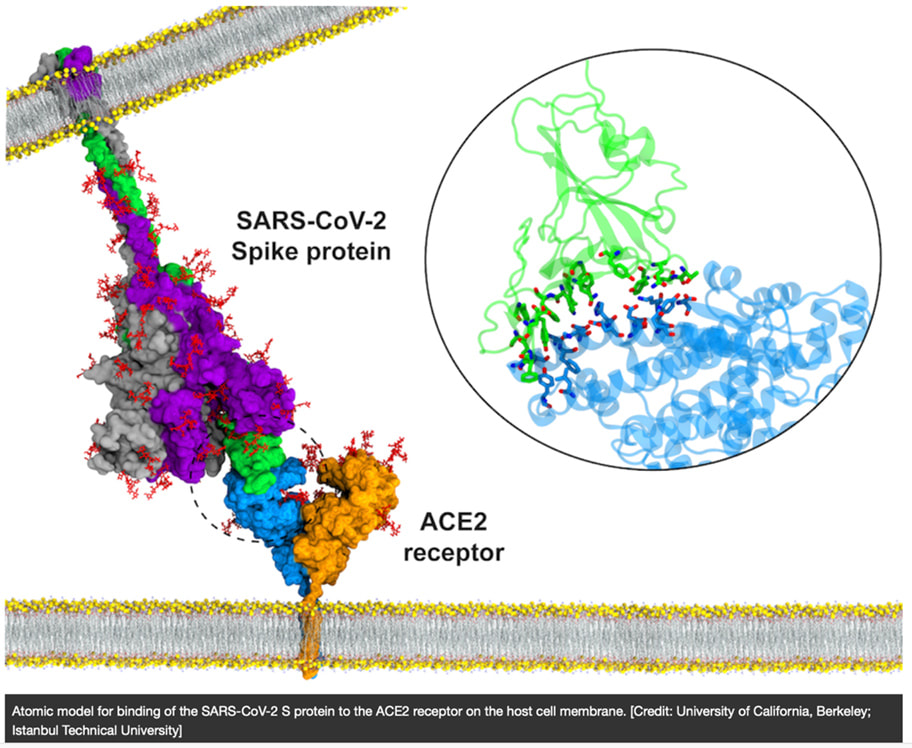
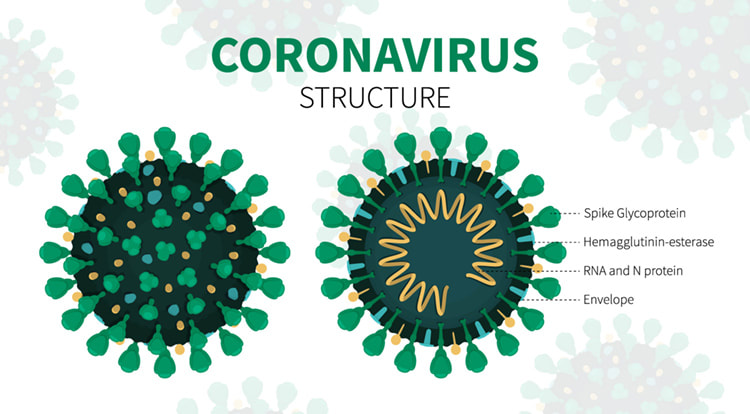
Many studies now have highlighted a significant association between gut microbiota alteration and persistent respiratory dysfunction in coronavirus disease 2019 (COVID-19) patients. One preprint publication reveals that altered diversity and composition of the gut microbiota together with increased plasma levels of lipopolysaccharide-binding protein (LBP) is associated with respiratory dysfunction in COVID-19 patients even after three months of hospitalization. Other research found that the gut microbiome of COVID-19 recovered patients returned to an uninfected status post-illness; findings in this small cohort, comprised of primarily African American patients, seemed to support the connection between SARS-CoV-2-mediated pathogenesis and the human gut microbiota. The presence of SARS-CoV-2 is associated with an increased relative abundance of Campylobacter, Klebsiella, two biological classes associated with gastrointestinal (GI) disease, while recovered patients displayed a microbiota composition similar to that of uninfected patients. As such, managing gastrointestinal distress may be key to minimizing the lasting effects of SARS-CoV-2 pathogenesis seen in many patients and will likely improve the prognosis and outcomes for acutely infected individuals. Increased Oxidative Stress We know that increases in oxidative stress lead to commensurate increases in inflammation that is associated with neurological and other disease. In this review, authors highlight the evidence seen in both acute COVID and ME/CFS of various underlying biological disorders. In particular, the researchers suggest a central role for the way cells behave when too many oxygen molecules pile up in a cell, causing oxidative stress or redox imbalance. The team describes how redox imbalance may be connected to the inflammation and disorders of metabolism that are found in the two diseases. 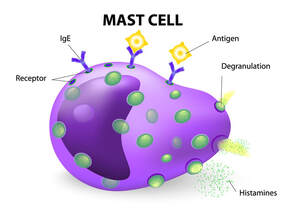 Mast Cell Activation Long COVID-19 patients are susceptible to endothelial dysfunction due to persistent immune activation part of which is due to mast cell activation. Endothelial dysfunction affects the heart whereby the large blood vessels constrict instead of opening, causing reduced blood flow. Damaged endothelial cells can also stimulate mast cells, a type of blood cell that’s part of the immune system. Their job is to defend against foreign bodies by releasing chemicals like histamines. Activated mast cells were recently found in autopsies of Covid-19 patients and are linked to clots and pulmonary edemas. In this study it was found that SARS-CoV-2 spike protein (S-protein) primes inflammatory cytokine secretion in macrophages derived from COVID-19 patients, but not in macrophages from healthy SARS-CoV-2 naïve individuals. The SARS-CoV-2 S-protein is a surface exposed and highly immunogenic viral component which induces a potent humoral immune response. Dr. Tina Peters, United Kingdom, studied over 2,000 long COVID sufferers and found that their profiles are strikingly similar to patients with mast cell activation syndrome (MCAS) and histamine intolerance and believes that COVID 19 hyper-inflammation and post-COVID illness may be rooted in MCAS. Hyperactive mast cells that drive inflammation and react to infectious and other pathogens can develop into a continuous activation loop, leading to cytokine storms. They also express angiotensin converting enzyme 2 (ACE2), now known as the principal receptor for SARS-CoV-2 spike protein, thereby defining a route by which mast cells could also become hosts for this virus and or for spike protein. Mast cells are present in all vascularized tissues, but are dominant at the environmental interfaces and in vessel walls. These hyper-inflammatory cytokine storms in many severely symptomatic COVID-19 patients may be rooted in an atypical response to SARS-CoV-2 by the dysfunctional mast cells of MCAS, rather than exhibiting a normal response by normal cells. Advanced senescence Advanced senescence may well be one of the many reasons why older patients, especially those who are frail and/or who have underlying medical problems, are most susceptible to COVID. We know that with aging comes a physiological decline of organ functions involving accumulation of cells that undergo a senescence state. Senescence is a process by which a cell ages and permanently stops dividing but does not die. Senescent cells are in a state of cell cycle arrest, remaining metabolically active and secreting a typical profile of inflammatory proteins resulting in chronic inflammation. This response can also trigger immune dysregulation. They also affect stem cell capability and shorten telomeres, which are repetitive sequences of non-coding DNA that protect the chromosome from damage. Senescent cells also have been implicated in the formation of misfolded proteins such as tau and amyloid-β, aggregations of which are associated with neurodegenerative disease such as Alzheimer’s and have been suggested to drive senescence themselves. And there are a number of recent articles that point to spike protein as the driver of senescence. What is Spike Protein? Spike protein binds to a surface receptor on our cells called ACE2, triggering uptake of the virus particle and eventually membrane fusion. But what is the ACE2 receptor?
It has been suggested that large concentrations of spike protein may bind to our ACE2 receptors and linger, blocking the regular functioning of these receptors in various tissues. The disruption of these receptors has been associated with a multitude of adverse effects through altered tissue functioning. The vaccines produced against COVID have utilized either mRNA or an adenovirus vector to direct human cells to produce the spike protein against which the body produces mostly neutralizing antibodies. When the COVID-19 vaccines were designed, it was not appreciated that the spike protein could potentially damage cells in the body. With each vaccine injection, billions of mRNA molecules wrapped in a lipid nanoparticle are injected into the body. It is important to note that mRNA has a half-life of around one to two weeks, depending on the mRNA, and during that interim, each mRNA molecule makes around 2,000 to 5,000 spike proteins. This will form trillions of spike proteins. The spike protein itself is damaging. S1, the binding protein for SARS-CoV-2, can like other binding proteins usually cause damage by themselves as they detach from the virus. They activate inflammation and resulting cardiovascular, hematological and neurological damage although multiple other organ systems can be impacted. Once in your blood circulation, the spike protein then binds to platelet receptors and the cells that line your blood vessels. When this occurs, platelets will clump together, resulting in blood clots and/or abnormal internal bleeding. Just a few ways that S1 Protein can damage the body and brain:
In my blog, I speak about the immune cross-reactivity that occurs with COVID; this work by Vojdani et al found that the COVID virus reacted with 28 out of 55 human tissues, many of which were found to be in the brain. Additional investigations include:
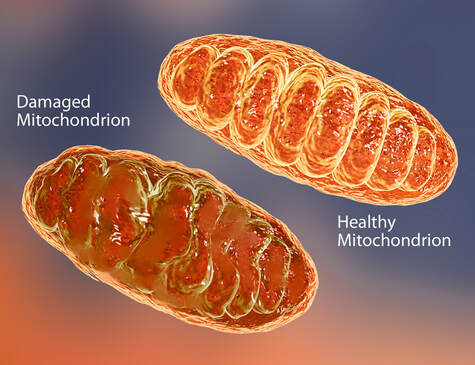
SARS-CoV-2 enters brain capillary endothelial cells via spike protein and in long haulers we are seeing a dysregulated immune response well after the detectable virus has been cleared. Dr. Bruce Patterson, CEO of IncellDX and part of the global team researching long COVID, identified the presence of spike protein (or a SARS-CoV-2 fragment) retained in the non-classical monocytes for up to 15 months. No evidence of residual intact virus was discovered in any of the patients. CREDIT: Matthias P. Nägele, Bernhard Haubner, Felix C. Tanner, Frank Ruschitzka, Andreas J. Flammer, Endothelial dysfunction in COVID-19: Current findings and therapeutic implications, Atherosclerosis, Volume 314, 2020, Pages 58-62, ISSN 0021-9150, https://doi.org/10.1016/j.atherosclerosis.2020.10.014. (https://www.sciencedirect.com/science/article/pii/S0021915020305815) Mitochondrial Injury and the Cell Danger Response Mitochondria are the powerhouses of the cell and are largely involved in maintaining cell immunity, homeostasis, and cell survival/death. SARS-CoV-2 uses its spike protein to bind to ACE2 receptors to eventually enter into cells. ACE2 influences mitochondrial functions, and a lack of ACE2 correlates with decreased ATP production; ATP is the principal molecule for storing and transferring energy in cells. When SARS-CoV-2 enters the cell via the ACE2 receptor, it sends its genetic material towards the mitochondria to influence ROS production, mitophagy, iron storage, platelet coagulability, and cytokine production stimulation. These functions are already compromised in aging patients and in those with comorbidities. As a result, we wind up with impaired mitochondrial functions that amplify other issues that contribute to severe outcomes, such as ferritin storage in diabetes and increased coagulability in heart disease. Under the influence of a viral infection, mitochondria experience a loss of integrity in structure and function and can trigger an immune response that activates the production of inflammasomes, which may contribute to autoimmunity and ongoing inflammatory reactions. Viral influence on mitochondrial activities can lead to altered energy levels by changing mitochondrial density and function, resulting in suboptimal energy output. In a 2020 article in Mitochondrion, Robert Naviaux, MD, PhD, discusses the cell danger response (CDR) and its connection to environmental health, mitochondrial function, and chronic illness. The CDR is a universal response to environmental threat, stress, infection or injury, and it is the mitochondria that sense and respond to changes in the cellular environment and mediate the regulators of the CDR that signal safety or danger within the cell. Once initiated, the CDR cannot be turned off and must cycle through to completion to effectively resolve the danger response. If the CDR abnormally persists, it is likely that whole body metabolism, the gut microbiome, and multiple organ systems will become impaired and chronic disease can emerge. Previous illness, stress, environmental toxins, chronic inflammatory illnesses, hormonal imbalances, autoimmune disorders, and allergies can impede the process of resolution of the CDR. Since much of the CDR is mediated by the mitochondria, it is crucial to support mitochondrial function, and foster general “good health habits” to promote a healthy CDR and potentially resolve chronic illness.
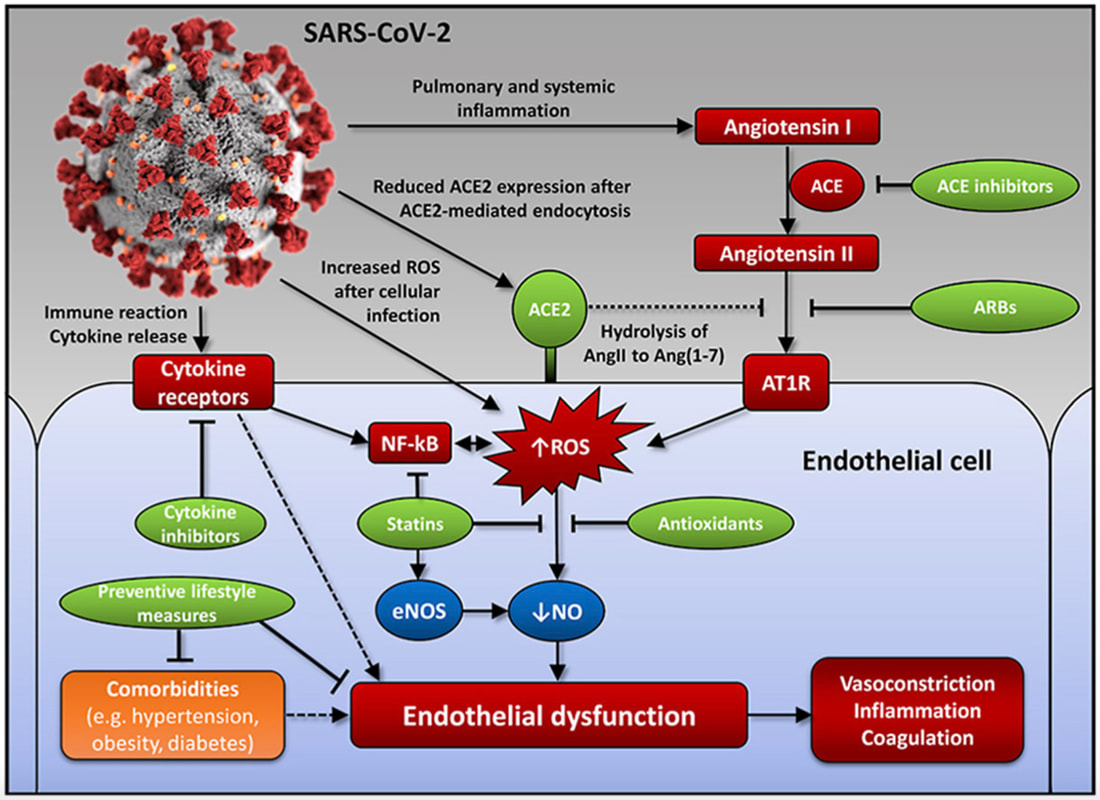
Alteration of Neurotransmitters In this study the authors identified a link between spike protein and the risk of neurodegeneration through interference with neurotransmitter sites, in this case, monoamine oxidase enzyme (MAO). What results is metabolic conversion and misbalancing of neurotransmitter levels with a depletion of serotonin, dopamine and norepinephrine. This event alone can increase the risk of neurodegenerative diseases such as Parkinson’s and Alzheimer’s, as well as neuropsychiatric symptoms. Endothelial Damage/Vascular Disease Additional research has shown that patients with long COVID syndrome continue to have higher measures of blood clotting, which may explain to some degree their persistent symptoms, such as reduced state of physical fitness, ongoing brain fog, and fatigue. The endothelium is a thin membrane that lines the inside of the heart and blood vessels and is an important part of the cardiovascular system. The endothelium function provides a critical interface between the blood compartment and tissues and helps maintain homeostasis. Endothelial cells release substances that control vascular relaxation and contraction as well as enzymes that control blood clotting, immune function and platelet (a colorless substance in the blood) adhesion. Brain microvascular endothelial cells (BMEC) are a central element of the microvasculature that forms the blood-brain barrier (BBB) and shields the brain against toxins and immune cells. One investigation found that clotting markers were significantly elevated in the blood of patients with long COVID syndrome compared with healthy controls. Researchers noted that these clotting markers were higher in patients who required hospitalization with their initial COVID-19 infection, but also found even patients who were able to manage their illness at home still had persistently high clotting markers. The scientists observed that “higher clotting was directly related to other symptoms of Long COVID syndrome, such as reduced physical fitness and fatigue. Even though markers of inflammation had all returned to normal levels, this increased clotting potential was still present in long COVID patients.” SARS-CoV-2 spike protein impairs endothelial function via downregulation of the ACE2 receptor A recent European study revealed that COVID-19 can kill brain cells known as endothelial cells. Several autopsy studies of patients who died of COVID-19 have revealed the presence severe endothelial damage and widespread vascular thrombosis, raising the possibility that this higher incidence of thromboembolic events might be due to a COVID-19-induced coagulopathy. In fact, autopsies have revealed how thrombosis is a prominent feature in multiple organs, despite full anticoagulation. Also concerning relevant to endothelial damage, in this study, Dr. Stephen Gundry found in looking at multiple protein biomarkers, which are predictable in assessing risk of acute coronary syndrome, that the mRNA vaccines dramatically increased inflammation of the endothelium and T cell infiltration of cardiac muscle. These findings may account for the observations of increased thrombosis, cardiomyopathy, and other vascular events following vaccination. Damage to DNA Emerging evidence is also showing that in the nucleus of our cells the spike protein impairs our cells’ ability to repair DNA. In this paper, they demonstrated that: 1. Segments of SARS-CoV-2 Viral RNA can become integrated into human genomic DNA. 2. This newly acquired viral sequence is not silent, meaning that these genetically modified regions of genomic DNA are transcriptionally active (DNA is being converted back into RNA). Exosomes Exosomes are small vesicles secreted from cells that participate in intercellular communication events. It’s believed that exosomes are involved in transmission of pathology and explain why COVID is so easily spread from person to person SARS-CoV-2 modulates the production and composition of exosomes, and can exploit exosome formation, secretion, and release pathways to promote infection, transmission, and intercellular spread. The thinking is that host exosome pathways are “hijacked” by viruses and that these virally modified exosomes can contribute to virus spread and immune evasion. Examples of physiological functions subject to regulation by exosomes Blood clotting Activation or suppression of immune responses Inflammation Tumor growth This paper discusses the detection of spike protein in persons vaccinated with the Pfizer mRNA vaccine. The most significant finding is that spike protein was present on exosomes for at least four months after the second injection. This surprisingly long persistence raises the prospect of sustained inflammation within and damage to organs which express the spike protein. We know that exosomes also can easily cross the blood-brain barrier (BBB); in this study, SARS-CoV-2 Spike protein and its role in manipulating exosomal cargo was examined with downstream targets in human microglia. The study provides novel and relevant insights regarding the impact of spike gene on shuttling of host microRNAs via exosomes to trigger the neuroinflammation. Exosomes and other extracellular vesicles have emerged as a potent mediators during host-pathogen interactions.* In the context of communication between periphery and the central nervous system (CNS) exosomes also have become instrumental and play a crucial role during CNS infection and neuroinflammation. Exosomes are key mediators of communication and waste management among neurons, glial cells and connective tissue during both physiological and pathological conditions in the brain. They also carry proteins like amyloid and alpha synuclein, tau, and other proteins that can promote pathology in the brain.  Groundbreaking[ work by Dr. Bruce Patterson in furthering our understanding. One scientist, Dr. Bruce Patterson, seems to have broken the code to truly understanding long COVID. Several points have emerged from his research including:
These cytokines, CCL5/RANTES, IL-2, IL-4, CCL3, IL-6, IL-10, IFN-γ, and VEGF, were significantly elevated (all P<0.001) while GM-CSF and CCL4 were significantly reduced in COVID patients in general. They found that long-haul COVID patients were characterized by increased IFN-γ and IL-2, and reduced CCL4 production. We know that TNF alpha is major marker of macrophage activation.
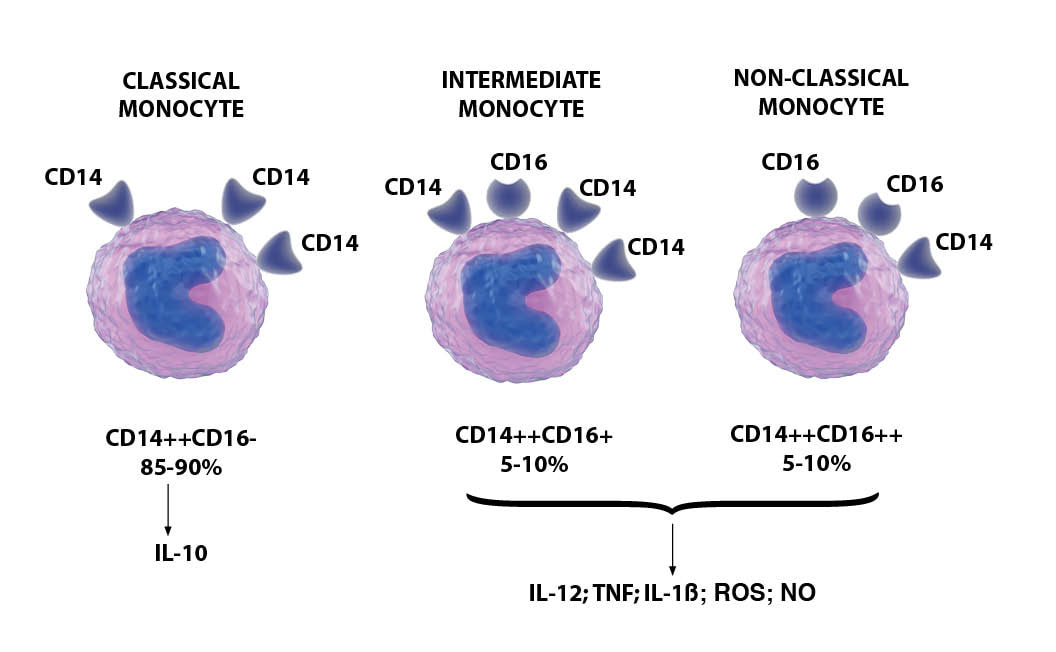
In the second paper, “Persistence of SARS CoV-2 S1 Protein in CD16+ Monocytes in Post-Acute Sequelae of COVID-19 (PASC) Up to 15 Months Post-Infection,” the team investigated the presence of SARS-CoV-2 S1 protein in 46 individuals. They analyzed different immune cells, including T-cell, B-cell, and monocytic subsets (monocytes are a type of white blood cell associated with presence of infection) in both severe COVID-19 patients and in patients with post-acute sequelae of COVID-19 (PASC), also known as long COVID. The levels of both intermediate (CD14+, CD16+) and non-classical monocyte (CD14Lo, CD16+) were significantly elevatedin PASC patients up to 15 months post-acute infection compared to healthy controls. These cells are highly inflammatory. Dr. Patterson’s paper discussing his findings in the vaccine-injured patients is expected to be in preprint January 2022. What are non-classical monocytes? Non-classical monocytes comprise about 2–11% of circulating monocytes. They are mobile in nature and patrol the endothelium in search of injury. They can have pro-inflammatory behavior and secrete inflammatory cytokines in response to infection; they are mostly involved in “trash cleanup” and the antiviral response. Interestingly, they also appear to be unique in the patrolling behavior they exhibit around the blood vessels. When they are drawn to blood vessels they can cause injury or can cause the blood vessels to inappropriately dilate. The monocyte binding also triggers the production of vascular endothelial growth factor (VEGF), a potent angiogenic factor, which then dilates the blood vessels. Fractalkine and RANTES/ CCR5 signals for harm: beacons in the eye of the storm. Non-classical monocytes are capable of causing inflammation throughout the body in response to a chemokine called fractalkine/CX3CL1 (chemokines are important factors for cellular recruitment into inflamed tissues) and RANTES/CCR5. CX3CL1 is abundantly expressed by activated endothelium and is an important regulator of many aspects of endothelial function and dysfunction, including thrombosis. When upregulated, it is contributing to a pro-thrombotic environment and immune cell recruitment Chemokines are important factors for cellular recruitment into inflamed tissues RANTES is a chemokine that is stored in the alpha granules of platelets that can be rapidly released after platelet activation RANTES binds to the chemokine receptor CCR5. CCR5 is a chemotactic molecule that can amplify inflammatory responses and is expressed on immune cells such as T-lymphocytes, macrophages, and NK cells. CCL5/RANTES acts like a magnet for these immune cells expressing CCR5. Thus, CCL5/RANTES is a marker for the early stages of immune dysregulation in COVID-19 and as a possible therapeutic marker to determine when and how long therapy targeting CCR5 or RANTES should be continued. Non-classical monocytes expressing high levels of CX3CR1 are involved in complement and Fc gamma-mediated phagocytosis and anti-viral responses. Think of fractalkine like a beacon in the light house…attracting and then attaching the non-classical, spike-laden monocytes to the endothelial lining of our blood vessels. Retained Spike Protein. Patterson’s study also noted another staggering finding: 73% of the non-classical monocytes showed retained spike protein up to 15 months out from the infection with COVID 19. A Perpetual Cycle of Injury. Why would these cells with the retained spike protein still be present for so long (up to 15 months) after clearance of the viral illness? In humans, the monocyte pool comprises three subsets (classical, intermediate, and non-classical) that circulate in dynamic equilibrium. The lifespan of a monocyte is typically only7-14 days. These non-classical monocytes are the only monocytes to carry the CX3CR1 receptor when it binds to fractalkine, turns on an anti-apoptotic (prevents cell death) protein that allows the monocytes to survive longer than usual. So essentially, they become the cells that seem to cause a perpetual cycle of damage. It also causes the monocytes to revert from their anti-inflammatory state and start pumping out pro-inflammatory cytokines. CX3CR1 is also an important player in getting the monocytes to engage in their vascular patrols and deleting CX3CR1 has been shown to reduce their patrolling behavior. The fractalkine receptor CX3CR1 is a key mediator of atherogenesis and damage to the vascular system. These monocytes express CCR5 and are mobilized by exercise which explains why symptoms of Long COVID can be made worse by exercise or sometimes even movement. The third striking discovery made by Dr Patterson was that whole viral genome sequencing revealed no evidence of retained virus. Given the continually evolving landscape of discovery regarding long COVID, this further complicates an already-complex situation. In short:
What about the vaccine injured? Dr. Patterson has found that in both long COVID and vaccine-injured patients, there is retained spike protein in this subset of monocytes. He has studied roughly 200 individuals who showed symptoms of long COVID in the months after being vaccinated. Machine-learning analyses indicated it was a quite heterogeneous group, but with one exception (no elevations of VEGF). Immunologically, they looked like long haulers and the long-hauler treatment worked for them. Therefore, it is to be assumed that the same pathophysiology in long COVID is also manifesting in those with injuries after the COVID vaccinations. This data as well indicates that the primary SARS-CoV-2 vaccine antigen (S-protein) selectively and potently drives pro-inflammatory cytokine secretion in human monocytes. Dr. Patterson’s next paper will discuss this in more detail. Testing The IncellKINE laboratory test examines the differences in cytokine responses between people with active cases of COVID-19 and long COVID patients. Using a blood test to measure immune markers and cytokines, researchers gain greater insight into the immune responses of long COVID patients. They can also definitively determine who has long COVID. The cost for the Cytokine 14 panel is $360, this includes shipping the kit to you by FedEx express saver/ground home delivery and shipping of the specimen back to Radiance by priority overnight, testing, and administrative charges. In our upcoming blog in this series, we will discuss Dr Patterson’s approach to treatment of the syndrome. While treatments will evolve commensurate to the continually updated situation, treatment protocols to date are strategically designed based on the results of the cytokine panel – much like so many other conditions, including neurological disorders, it is imperative to first restore the immune system to a less inflammatory state. In hope and healing, Dr. Suzanne Gazda References: Low, R. N., Low, R. J., & Akrami, A. (2020, November 12). A Cytokine-based model for the pathophysiology of Long COVID symptoms. https://doi.org/10.31219/osf.io/7gcnv Proal Amy D., VanElzakker Michael B. Long COVID or Post-acute Sequelae of COVID-19 (PASC): An Overview of Biological Factors That May Contribute to Persistent Symptoms. Frontiers in Microbiology. (2021) Volume 12. https://www.frontiersin.org/article/10.3389/fmicb.2021.698169 Wang, E.Y., Mao, T., Klein, J. et al. Diverse functional autoantibodies in patients with COVID-19. Nature 595, 283–288 (2021). https://doi.org/10.1038/s41586-021-03631-y Vasilevska, V., Guest, P.C., Bernstein, HG. et al. Molecular mimicry of NMDA receptors may contribute to neuropsychiatric symptoms in severe COVID-19 cases. J Neuroinflammation 18, 245 (2021). https://doi.org/10.1186/s12974-021-02293-x Domínguez-Andrés J, et al. The BNT162b2 mRNA vaccine against SARS-CoV-2 reprograms both adaptive and innate immune responses. medRxiv, 2021. doi: https://doi.org/10.1101/2021.05.03.21256520, https://www.medrxiv.org/content/10.1101/2021.05.03.21256520v1 Gazda, S. (2020) Does all disease begin in the gut? Gazda Integrative Neurology. https://www.suzannegazdamd.com/blog/does-all-disease-begin-in-the-gut Rachel C. Newsome, Josee Gauthier, Maria C. Hernandez, George E. Abraham, Tanya O. Robinson, Haley B. Williams, Meredith Sloan, Anna Owings, Hannah Laird, Taylor Christian, Yilianys Pride, Kenneth J. Wilson, Mohammad Hasan, Adam Parker, Michal Senitko, Sarah C. Glover, Raad Z. Gharaibeh & Christian Jobin (2021) The gut microbiome of COVID-19 recovered patients returns to uninfected status in a minority-dominated United States cohort, Gut Microbes, 13:1, DOI: 10.1080/19490976.2021.1926840 Bindu D. Paul, Marian D. Lemle, Anthony L. Komaroff, Solomon H. Snyder. Redox imbalance links COVID-19 and myalgic encephalomyelitis/chronic fatigue syndrome. Proceedings of the National Academy of Sciences. August 2021, 118 (34) e2024358118; DOI: 10.1073/pnas.2024358118 Theoharis C. Theoharides, Potential association of mast cells with coronavirus disease 2019, Annals of Allergy, Asthma & Immunology, Volume 126, Issue 3, 2021, Pages 217-218, ISSN 1081-1206, https://doi.org/10.1016/j.anai.2020.11.003. https://www.sciencedirect.com/science/article/pii/S1081120620311650 https://www.annallergy.org/article/S1081-1206(20)31165-0/fulltext#bib4 Sebastian J Theobald, Alexander Simonis, Theodoros Georgomanolis, et al. Long-lived macrophage reprogramming drives spike protein-mediated inflammasome activation in COVID-19. EMBO Mol Med (2021) 13: e14150. https://www.embopress.org/doi/full/10.15252/emmm.202114150 Lawrence B. Afrin, Leonard B. Weinstock, Gerhard J. Molderings Covid-19 hyperinflammation and post-Covid-19 illness may be rooted in mast cell activation syndrome. International Journal of Infectious Diseases. 2021. https://www.ijidonline.com/article/S1201-9712(20)30732-3/fulltext Gazda, S. Alpha Synuclein in neurodegenerative disease. (2020) https://www.suzannegazdamd.com/scientifically-speaking1/alpha-synuclein-in-neurodegenerative-disease https://www.biorxiv.org/content/10.1101/2021.01.02.424917v2.full SARS-CoV-2 infects lung epithelial cells and induces senescence and an inflammatory response in patients with severe COVID-19 https://journals.asm.org/doi/10.1128/JVI.00794-21 SARS-CoV-2 Spike Protein Induces Paracrine Senescence and Leukocyte Adhesion in Endothelial Ashraf UM, Abokor AA, Edwards JM, et al. SARS-CoV-2, ACE2 expression, and systemic organ invasion. Physiol Genomics. 2021;53(2):51-60. doi:10.1152/physiolgenomics.00087.2020 Rhea, E.M., Logsdon, A.F., Hansen, K.M. et al. The S1 protein of SARS-CoV-2 crosses the blood–brain barrier in mice. Nat Neurosci 24, 368–378 (2021). https://doi.org/10.1038/s41593-020-00771-8;https://www.nature.com/articles/s41593-020-00771-8 Reaction of Human Monoclonal Antibodies to SARS-CoV-2 Proteins With Tissue Antigens: Implications for Autoimmune Diseases https://www.frontiersin.org/articles/10.3389/fimmu.2020.617089/full?fbclid=IwAR2omo1nx2CfIaV2wXondJdDzwwVbN3g2D7LOUfJC-t4oPAo_D3hNHpS1S4 https://rightsfreedoms.wordpress.com/2021/06/03/confidential-pfizer-research-document/ Dr. Bruce Patterson https://covidlonghaulers.com/ https://incelldx.com/ ZRT Laboratory https://www.zrtlab.com/blog/archive/long-covid-and-mitochondrial-dysregulation/#T5 Naviaux R. K. (2020). Perspective: Cell danger response Biology-The new science that connects environmental health with mitochondria and the rising tide of chronic illness. Mitochondrion, 51, 40–45. https://doi.org/10.1016/j.mito.2019.12.005 https://www.preprints.org/manuscript/202003.0422/v1 Patterson BK, Guevara-Coto J, Yogendra R, Francisco EB, Long E, Pise A, Rodrigues H, Parikh P, Mora J and Mora-Rodríguez RA (2021) Immune-Based Prediction of COVID-19 Severity and Chronicity Decoded Using Machine Learning. Front. Immunol. 12:700782. doi: 10.3389/fimmu.2021.700782 https://www.frontiersin.org/articles/10.3389/fimmu.2021.700782/full https://www.frontiersin.org/articles/10.3389/fimmu.2021.746021/abstract Tetz, G.; Tetz, V. SARS-CoV-2 Prion-Like Domains in Spike Proteins Enable Higher Affinity to ACE2. Preprints 2020, 2020030422 (doi: 10.20944/preprints202003.0422.v1) https://www.biorxiv.org/content/10.1101/2021.08.30.458208v1.full.pdf https://www.rcsi.com/dublin/news-and-events/news/news-article/2021/08/blood-clotting-may-be-the-root-cause-of-long-covid-syndrome Fogarty, H, Townsend, L, Morrin, H, et al; the Irish COVID-19 Vasculopathy Study (iCVS) investigators. Persistent endotheliopathy in the pathogenesis of long COVID syndrome. J Thromb Haemost. 2021; 19: 2546– 2553. https://doi.org/10.1111/jth.15490 Wenzel, J., Lampe, J., Müller-Fielitz, H. et al. The SARS-CoV-2 main protease Mpro causes microvascular brain pathology by cleaving NEMO in brain endothelial cells. Nat Neurosci 24, 1522–1533 (2021). https://doi.org/10.1038/s41593-021-00926-1 Maximilian Ackermann, M.D., Stijn E. Verleden, Ph.D., Mark Kuehnel, Ph.D., Axel Haverich, M.D., et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N Engl J Med 2020; 383:120-128. DOI: 10.1056/NEJMoa2015432. https://www.nejm.org/doi/full/10.1056/nejmoa2015432 Wichmann D, Sperhake JP, Lütgehetmann M, et al. Autopsy Findings and Venous Thromboembolism in Patients With COVID-19: A Prospective Cohort Study. Ann Intern Med. 2020;173(4):268-277. doi:10.7326/M20-2003 Steven R Gundry. Abstract 10712: Mrna COVID Vaccines Dramatically Increase Endothelial Inflammatory Markers and ACS Risk as Measured by the PULS Cardiac Test: a Warning. https://www.ahajournals.org/doi/10.1161/circ.144.suppl_1.10712 Jiang, H., & Mei, Y. F. (2021). SARS-CoV-2 Spike Impairs DNA Damage Repair and Inhibits V(D)J Recombination In Vitro. Viruses, 13(10), 2056. https://doi.org/10.3390/v13102056 Bansal S, Perincheri S, Fleming T, et al. Cutting Edge: Circulating Exosomes with COVID Spike Protein Are Induced by BNT162b2 (Pfizer-BioNTech) Vaccination prior to Development of Antibodies: A Novel Mechanism for Immune Activation by mRNA Vaccines. J Immunol. 2021;207(10):2405-2410. doi:10.4049/jimmunol.2100637. https://pubmed.ncbi.nlm.nih.gov/34654691/ Mishra R, Banerjea AC. SARS-CoV-2 Spike Targets USP33-IRF9 Axis via Exosomal miR-148a to Activate Human Microglia. Front Immunol. 2021;12:656700. Published 2021 Apr 14. doi:10.3389/fimmu.2021.656700. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8079643/ Barberis Elettra, Vanella Virginia V., Falasca Marco, Caneapero Valeria, Cappellano Giuseppe, et al. Circulating Exosomes Are Strongly Involved in SARS-CoV-2 Infection. Frontiers in Molecular Biosciences. (2021). Vol. 8. DOI=10.3389/fmolb.2021.632290 https://www.frontiersin.org/articles/10.3389/fmolb.2021.632290/full *See also: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8079643/#B32 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8079643/#B41 Patterson BK, Guevara-Coto J, Yogendra R, et al. Immune-Based Prediction of COVID-19 Severity and Chronicity Decoded Using Machine Learning. Front Immunol. 2021;12:700782. Published 2021 Jun 28. doi:10.3389/fimmu.2021.700782 Patterson BK, et al. Persistence of SARS CoV-2 S1 Protein in CD16+ Monocytes in Post-Acute Sequelae of COVID-19 (PASC) Up to 15 Months Post-Infection Summary: SARS CoV-2 S1 Protein in CD16+ Monocytes In PASC. Frontiers in Immunology. (2021) https://www.frontiersin.org/articles/10.3389/fimmu.2021.746021/abstract https://onlinelibrary.wiley.com/doi/full/10.1002/cyto.a.23280 https://onlinelibrary.wiley.com/doi/full/10.1002/cyto.a.23280 Barberis Elettra, Vanella Virginia V., Falasca Marco, Caneapero Valeria et al. Long-lived macrophage reprogramming drives spike protein-mediated inflammasome activation in COVID-19. EMBO Mol Med (2021) 13: e14150.https://doi.org/10.15252/emmm.202114150
0 Comments
Your comment will be posted after it is approved.
Leave a Reply. |
AuthorSuzanne Gazda M.D. Neurologist Archives
January 2024
Categories |
||||||||||||||||




 RSS Feed
RSS Feed