|
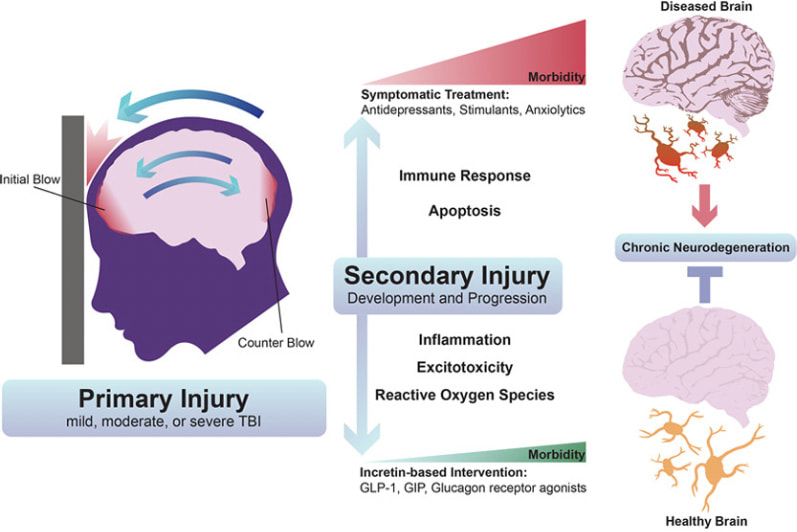
As I have noted in my ongoing long COVID series, the impacts to the brain post infection and post-vaccine injury can have long lingering neurological consequences. The symptoms may vary from mild to significant, regardless of the severity of the original illness. And while each patient may have different needs unique to their individual symptoms, the scientific community and clinicians are continuing to identify ways to address and treat these impacts. As we’ll discuss further in this article, intravenous immunoglobulin (IVIG) therapy which is a well-documented treatment in many autoimmune and other diseases, may also have value for use in some long COVID patients. Some background. The Centers for Disease Control and Prevention (CDC) estimates that one in five patients may suffer with the novel disorder of long COVID. But a recent study from the United Kingdom indicated nearly half of COVID-19 survivors from around the world still had symptoms an average of four months later, regardless of whether they had been hospitalized. Many, many have been injured as well from the COVID vaccines; I also have discussed in prior blogs the numerous mechanisms of injury in long COVID and post-vaccine injuries. For our complete long COVID series and additional titles, see: https://www.suzannegazdamd.com/blog---long-covid This study published by Philip Oldfield in January 2022, is an excellent review on how SARS CoV 2 affects the brain, with an emphasis on the role of the spike protein in patients with neurological symptoms. In other research, investigators were able to demonstrate a “novel pathogenic mechanism for the COVID-19-associated neurological symptoms that involves glia activation and non-cell autonomous hippocampal neuronal death by the brain-infiltrating S1 protein. In a paper published in October 2022 in the journal Cell, the findings showed high levels of neuroinflammation driven by chemical messengers called cytokines, with damage to cells in the brain that make myelin (oligodendrocytes) white matter; the white matter are described as the lines of communication in the central nervous system (CNS). Myelin is a protective coating that helps nerve transmission be more effective and efficient. This study also found damage to the hippocampus or the primary memory center of the brain. Most research has shown no evidence of lingering virus or, at least, no whole viral genome; but lingering viral remnants like spike protein have been uncovered in various tissues including the brain. The brain assumes the brunt of the impacts. I have discussed at length the “fallout” to the brain and CNS following a COVID infection and in some cases post vaccine. There is even a new term that has been assigned to these impacts, “Post COVID Neurological Syndrome.” What we are seeing and experiencing is truly a neurological crisis. In this yearlong study conducted by the Veterans Administration, long-term neurologic outcomes of COVID-19 were seen in 7% of patients post COVID regardless of age. We are seeing a tsunami of rising disability and for many the lingering cognitive issues are devastating. British scientists who studied medical records from around the world reported in the journal The Lancet Psychiatry in August that people who recovered from COVID had a higher risk of problems with their thinking and dementia even after two years had passed. Another 2022 study, published in the journal JAMA Neurology, looked at older COVID-19 patients for a year after they were discharged from hospitals in Wuhan, China. Compared with uninfected people, those who survived a severe case of COVID-19 were at higher risk for early onset, late-onset, and progressive decline in their thinking skills People who contracted COVID were twice as likely to receive a diagnosis of Alzheimer’s disease in the 12 months after infection, compared to those who didn’t get COVID, according to a study published in September 2022 in the journal Nature, which analyzed the health care databases of the U.S. Department of Veterans Affairs. In a study published by Wang et al in the Journal of Alzheimer’s Disease, researchers report that people 65 and older who contracted COVID-19 were more prone to developing Alzheimer’s disease in the year following their COVID diagnosis. The highest risk was observed in women at least 85 years old. In the UK biobank study, more generalized brain atrophy was noted in those following a COVID infection, with most patients reported as having had only a mild respiratory illness. Yet, the post-COVID patients also showed on the average a larger cognitive decline. Healing the broken brain. To help heal the brain, we must address high levels of neuroinflammation, microglial cell activation, excess oxidative stress, high levels of pro-inflammatory cytokines, mitochondrial dysfunction, micro clotting, restore healthy immune system balance and reduce / prevent prion formation and more. And we must find ways to shore up the blood-brain barrier (BBB) and provide beneficial realms of neuroplasticity protocols. Although treatment strategies are unique to each patient, we must look for all available methods to reduce the neurological implications and improve outcomes. The benefits of IVIG in brain health. In this recent paper, researchers explored the therapeutic benefit of IVIG in a controlled cortical impact mouse model of traumatic brain injury (TBI). TBI is a model that can teach us about activation of microglia and the downstream effects including a risk of future neurodegenerations. TBI induces a complex cascade of inflammatory events, which involves the local activation of resident microglia and astrocytes in the central nervous system (CNS), and the recruitment of peripheral immune cells to the lesion site. This inflammatory response is typically non-resolving and believed to contribute to the secondary degeneration of neural tissue that was otherwise spared during the initial insult. The hippocampus is known to be particularly vulnerable to secondary degeneration after brain trauma, with injury-induced neuropathological changes resulting in spatial learning and memory deficits. For example, IVIG:
Other ways IVIG offers benefits.
While we certainly know more about COVID than we may have when this pandemic first began almost three years ago, there is much still to be learned as events continue to evolve and new information comes to light. We have learned as well that the COVID vaccines themselves can impair immune health. A recent study showed an imbalance of immune sets with more IgG 4 spike autoantibodies. This has the potential to promote immune tolerance to the virus and may set the patient up to having a hard time clearing the virus and worse infection outcomes. The COVID vaccines also have been shown to alter innate and adaptive immunity (the two arms of the immune system), inhibit toll-like receptors needed to fight infections, and alter DNA repair mechanisms of injury. IVIG and long COVID. Could IVIG in the right clinical setting be beneficial in at least some long COVID patients? This approach does seem to offer promise in certain cases, and research outcomes certainly warrant further study to identify who could benefit most from including this protocol as part of a comprehensive treatment plan. The key for anyone suffering the challenges of a long COVID diagnosis is to work with a clinician experienced in treating the disorder and staying up to date on the continually evolving therapeutic options. For more information about IVIG and the immunological nature of long COVID, see: https://www.suzannegazdamd.com/blog---long-covid/the-immune-system-is-on-high-alert https://www.suzannegazdamd.com/blog/ivig-in-autoimmune-disease-therapies As always, please reach out to our offices if we can help or provide any additional guidance. In hope and healing, Dr. Suzanne Gazda References: O’Mahoney, L., Routen, A., et al. The prevalence and long-term health effects of Long Covid among hospitalised and non-hospitalised populations: A systematic review and meta-analysis. The Lancet. (2022) https://www.thelancet.com/journals/eclinm/article/PIIS2589-5370(22)00491-6/fulltext Chatterjee, A., Chakravarty, A. Neurological Complications Following COVID-19 Vaccination. Curr Neurol Neurosci Rep(2022). https://doi.org/10.1007/s11910-022-01247-x https://link.springer.com/article/10.1007/s11910-022-01247-x Oldfield PR, Hibberd J, Bridle BW. How Does Severe Acute Respiratory Syndrome-Coronavirus-2 Affect the Brain and Its Implications for the Vaccines Currently in Use. Vaccines (Basel). 2021;10(1):1. Published 2021 Dec 21. doi:10.3390/vaccines10010001 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8780773/ Oh J, Cho WH, Barcelon E, Kim KH, Hong J, Lee SJ. SARS-CoV-2 spike protein induces cognitive deficit and anxiety-like behavior in mouse via non-cell autonomous hippocampal neuronal death. Sci Rep. 2022;12(1):5496. Published 2022 Mar 31. doi:10.1038/s41598-022-09410-7 https://pubmed.ncbi.nlm.nih.gov/35361832/ Monje, M., Iwasaki, A. The neurobiology of long COVID. Cell. (2022) https://www.cell.com/neuron/fulltext/S0896-6273(22)00910-2 Ali, S.T., Kang, A.K., Patel, T.R., Clark, J.R., Perez-Giraldo, G.S., Orban, Z.S., Lim, P.H., Jimenez, M., Graham, E.L., Batra, A., Liotta, E.M. and Koralnik, I.J. (2022), Evolution of neurologic symptoms in non-hospitalized COVID-19 “long haulers”. Ann Clin Transl Neurol, 9: 950-961. https://doi.org/10.1002/acn3.51570. Xu, E., Xie, Y. & Al-Aly, Z. Long-term neurologic outcomes of COVID-19. Nat Med 28, 2406–2415 (2022). https://doi.org/10.1038/s41591-022-02001-z Taquet, M. et al. Neurological and psychiatric risk trajectories after SARS-CoV-2 infection: an analysis of 2-year retrospective cohort studies including 1 284 437 patients. The Lancet. 2022. https://www.thelancet.com/journals/lanpsy/article/PIIS2215-0366(22)00260-7/fulltext#%20 Wang L, Davis PB, Volkow ND, Berger NA, Kaelber DC, Xu R. Association of COVID-19 with New-Onset Alzheimer's Disease. J Alzheimers Dis. 2022;89(2):411-414. doi:10.3233/JAD-220717 https://pubmed.ncbi.nlm.nih.gov/35912749/ Douaud, G., Lee, S., Alfaro-Almagro, F. et al. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature 604, 697–707 (2022). https://doi.org/10.1038/s41586-022-04569-5. https://www.nature.com/articles/s41586-022-04569-5 Jiang Hu et al. SARS-CoV-2 Spike Impairs DNA Damage Repair and Inhibits V(D)J Recombination In Vitro. Viruses 2021 Oct 13;13(10):2056. Das M, Karnam A, Stephen-Victor E, et al. Intravenous immunoglobulin mediates anti-inflammatory effects in peripheral blood mononuclear cells by inducing autophagy. Cell Death Dis. 2020;11(1):50. Published 2020 Jan 23. doi:10.1038/s41419-020-2249-y https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6978335/ Istrin, G., Bosis, E. and Solomon, B. (2006), Intravenous immunoglobulin enhances the clearance of fibrillar amyloid-β peptide. J. Neurosci. Res., 84: 434-443. https://doi.org/10.1002/jnr.20886 Kazatchkine MD, Kaveri SV. Immunomodulation of autoimmune and inflammatory diseases with intravenous immune globulin. N Engl J Med. 2001;345(10):747-755. doi:10.1056/NEJMra993360. https://pubmed.ncbi.nlm.nih.gov/11547745/ Durandy A, Kaveri SV, Kuijpers TW, et al. Intravenous immunoglobulins--understanding properties and mechanisms. Clin Exp Immunol. 2009;158 Suppl 1(Suppl 1):2-13. doi:10.1111/j.1365-2249.2009.04022.x Chen, M., Arumugam, T., Leanage, G. et al. Disease-modifying effect of intravenous immunoglobulin in an experimental model of epilepsy. Sci Rep 7, 40528 (2017). https://doi.org/10.1038/srep40528 Additional references: Alden, M. et al. Intracellular Reverse Transcription of Pfizer BioNTech COVID-19 mRNA Vaccine BNT162b2 In Vitro in Human Liver Cell Line. MDPI. (2022) https://www.mdpi.com/1467-3045/44/3/73 Liu S, Hossinger A, Heumüller SE, et al. Highly efficient intercellular spreading of protein misfolding mediated by viral ligand-receptor interactions. Nat Commun. 2021;12(1):5739. Published 2021 Oct 19. doi:10.1038/s41467-021-25855-2 Rodríguez Y, Novelli L, Rojas M, et al. Autoinflammatory and autoimmune conditions at the crossroad of COVID-19. J Autoimmun. 2020;114:102506. doi:10.1016/j.jaut.2020.102506 Wang EY, Mao T, Klein J, et al. Diverse Functional Autoantibodies in Patients with COVID-19. Preprint. medRxiv. 2021;2020.12.10.20247205. Published 2021 Feb 1. doi:10.1101/2020.12.10.20247205 Murphy WJ, Longo DL. A Possible Role for Anti-idiotype Antibodies in SARS-CoV-2 Infection and Vaccination. N Engl J Med. 2022;386(4):394-396. doi:10.1056/NEJMcibr2113694 Chen Y, Xu Z, Wang P, et al. New-onset autoimmune phenomena post-COVID-19 vaccination. Immunology. 2022;165(4):386-401. doi:10.1111/imm.13443 Murata K, Nakao N, Ishiuchi N, et al. Four cases of cytokine storm after COVID-19 vaccination: Case report. Front Immunol. 2022;13:967226. Published 2022 Aug 15. doi:10.3389/fimmu.2022.967226 Qin Z, Bouteau A, Herbst C, Igyártó BZ. Pre-exposure to mRNA-LNP inhibits adaptive immune responses and alters innate immune fitness in an inheritable fashion. Preprint. bioRxiv. 2022;2022.03.16.484616. Published 2022 Aug 20. doi:10.1101/2022.03.16.484616 Nyström S, Hammarström P. Amyloidogenesis of SARS-CoV-2 Spike Protein. J Am Chem Soc. 2022;144(20):8945-8950. doi:10.1021/jacs.2c03925 Seth P, Sarkar N. A comprehensive mini-review on amyloidogenesis of different SARS-CoV-2 proteins and its effect on amyloid formation in various host proteins. 3 Biotech. 2022;12(11):322. doi:10.1007/s13205-022-03390-1 Fann DY, Lee SY, Manzanero S, et al. Intravenous immunoglobulin suppresses NLRP1 and NLRP3 inflammasome-mediated neuronal death in ischemic stroke. Cell Death Dis. 2013;4(9):e790. Published 2013 Sep 5. doi:10.1038/cddis.2013.326 Monje M, Iwasaki A. The neurobiology of long COVID. Neuron. 2022;110(21):3484-3496. doi:10.1016/j.neuron.2022.10.006 Irrgang P, Gerling J, Kocher K, et al. Class switch towards non-inflammatory, spike-specific IgG4 antibodies after repeated SARS-CoV-2 mRNA vaccination [published online ahead of print, 2022 Dec 22]. Sci Immunol. 2022;eade2798. doi:10.1126/sciimmunol.ade2798 Xu E, Xie Y, Al-Aly Z. Long-term neurologic outcomes of COVID-19. Nat Med. 2022;28(11):2406-2415. doi:10.1038/s41591-022-02001-z Chatterjee, A., Chakravarty, A. Neurological Complications Following COVID-19 Vaccination. Curr Neurol Neurosci Rep(2022). https://doi.org/10.1007/s11910-022-01247-x
1 Comment
Danielle
1/17/2023 02:24:07 pm
What are your thoughts on IVIG medications containing Spike Protein from the vaccinated donors? IVIG filters down to 20 to 30 nanometers and the spike alone without the enveloped virus is only 5 to 9 nanometers. Do you think there is a way to figure out if IVIG contains damaging spike protein?(Not the antibodies) The actual spike that is being created by vaccinated donors. Do you think IVIG is still helpful or could it be causing harm?
Reply
Your comment will be posted after it is approved.
Leave a Reply. |
AuthorSuzanne Gazda M.D. Neurologist Archives
January 2024
Categories |

 RSS Feed
RSS Feed