|
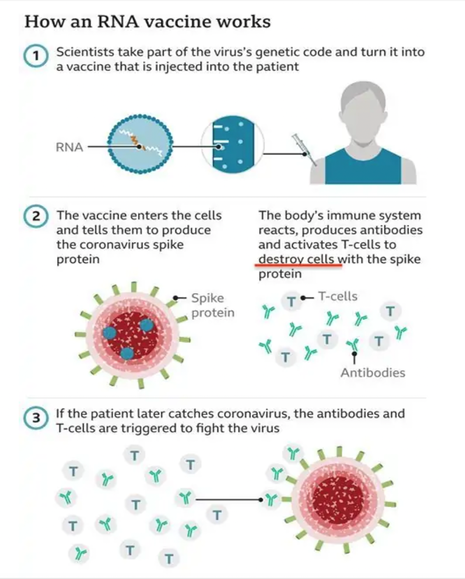
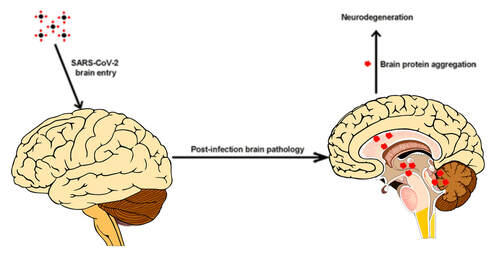
As I have discussed in detail in my previous blog in our long COVID series, residual virus is not thought to be the problem we face relevant to ongoing neurological issues following COVID infection. Medical News Today reported in a new perspective article, based on clinical observations, autopsies, and lab findings by a team of researchers in Chicago, which argues against the virus directly affecting brain cells. Instead, it appears that the brain is affected by inflammation, vascular damage, risk of clotting and immune dysregulation including molecular mimicry and the production of autoantibodies. Leftover viral fragments likely are to blame, with spike protein being the front runner on that list. Spike protein is a viral fragment and is the end product of the COVID vaccines. This protein is now being looked at as a biomarker for long COVID and has been found in the blood of those suffering with related symptoms for up to 12 months. Dr. Patterson’s findings of S 1 protein in monocytes (a type of WBC) for up to 15 months and high levels of inflammatory cytokines in long COVID and in a subset of post-vaccine-injured patients brings discoveries to the mystery of long COVID. Spike protein lingers in exosomes up to 4 months after vaccination. The big question is why does this toxic protein linger? We have never before seen a virus like COVID 19. And it’s important to note as well that we have never before employed in mass to humans, a vaccine utilizing genetically modified mRNA that sends a genetic code to create trillions of potentially damaging spike proteins. So, the “unknown” element is worrisome. We simply have no idea what the long term risks will be. COVID vaccines and how they generate spike protein As noted in some settings, over the last few months there have been multiple studies and articles appearing in peer-reviewed and other scientific journals that discuss the potentially serious side effects occurring in some patients following vaccination. The vaccine places a novel genetic code, i.e. mRNA molecule, in or on the surface of the host cell and sends a message to generate the production of the Wuhan spike protein. Antibodies are produced against the spike protein so that in the future the immune system can respond quickly to future COVID 19 infections. All the injections unleash genetic modification so that every cell is given a code to become a factory for spike protein production. Unfortunately, researchers have come to learn that the mRNA vaccines do not stay in the shoulder muscle after injection. In fact, they have the potential to spread far and wide throughout the body via the blood. A recent report shows that the COVID spike protein gets into the blood where it circulates for several days post-vaccination and then accumulates in organs and tissues, including the spleen, bone marrow, the liver, adrenal glands and in “quite high concentrations” in the ovaries. In a publication that reported on 13 young healthcare workers who received the Moderna vaccine. Researchers found detectable levels of SARS-CoV-2 protein in 11 of the 13 participants one day after first vaccination. “Spike protein was detectable in three of 13 participants for an average of 15 days after the first injection… for one individual, spike was detected at day 29”, circulating in the blood. These findings are very concerning and require further urgent study. As of this writing, according to the Vaccine Adverse Event Reporting System (https://vaersanalysis.info/), there are over 1.3 million reported adverse events, although this may be underrepresented. VAERS is a publicly accessible platform operated by the Centers for Disease Control and Prevention (CDC) to analyze reports of possible vaccine side effects; note that anyone can report an adverse event to VAERS. The problem with VAERS is not over reporting, but under reporting. Over a year into the biggest vaccination campaign in history, more than 12.3 billion doses have been administered across 184 countries, according to data collected by Bloomberg. The latest rate was roughly 12.5 million doses a day(as of July 31, 2022). In the US, 599 million doses have been given so far. During the last week, shots were administered at an average rate of 96,733 doses a day. There are no long-term safety studies for this brand-new technology. We are part of the ongoing discovery. Long COVID has become ‘the pandemic upon the pandemic’ and we see no end in sight to the rising numbers affected We are facing an eponymous tidal wave of health problems and are standing in the path of a tsunami, the might of which has never been seen. Please see my blog which discusses the very complicated nature of Long COVID “Why does long COVID happen?” https://www.suzannegazdamd.com/blog---long-covid/why-does-long-covid-happen Concerning mechanisms. We saw after the Spanish flu pandemic of 1918 an outbreak of a neurological condition called encephalitis lethargica, and subsequently many cases of post-encephalitic Parkinsonism. I want to point out that many of the mechanisms leading to Long COVID (including vaccine injuries) are the same factors that can fuel neurodegeneration. These considerations include:
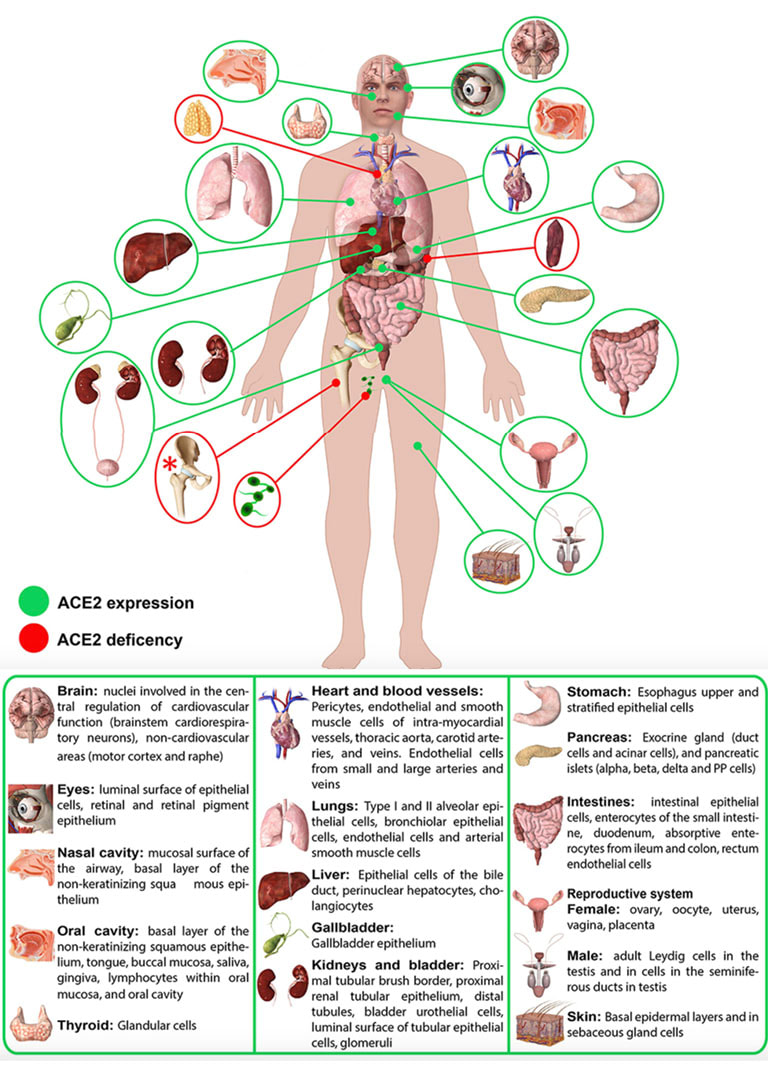
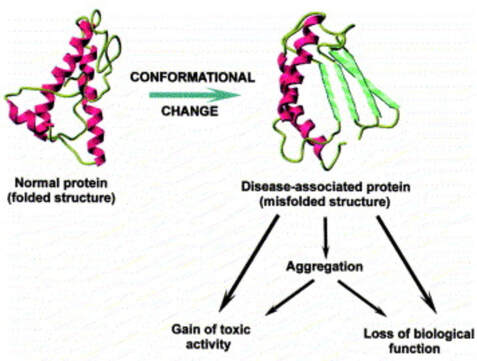
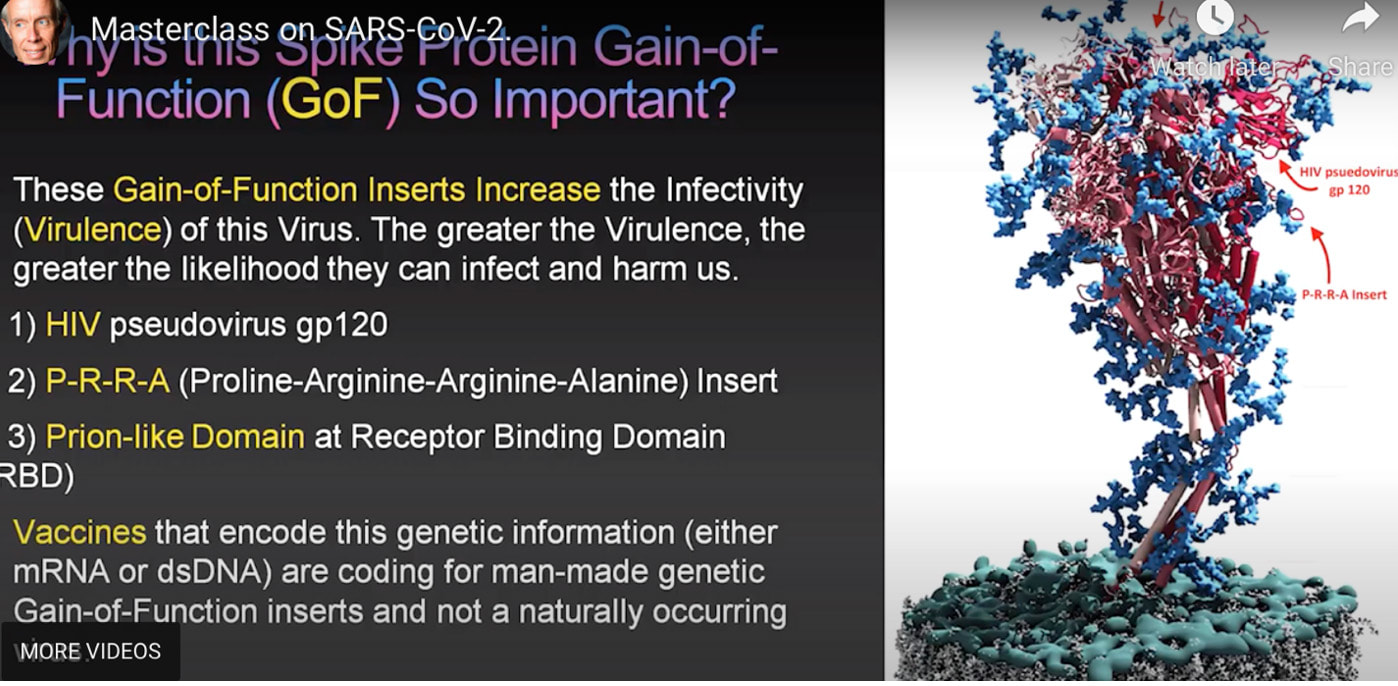
How these mechanisms of long COVID and Neurodegenerative disease are linked. ACE 2 Receptors. ACE 2 receptors are the binding sites for Spike protein and ACE2 plays a role in NDD. ACE2 receptors act like locks on cells, and the SARS-CoV-2 spike proteins act like keys that open the locks letting the virus enter cells to rapidly multiply. The ACE 2 receptors are all over the body and the brain and these receptors can respond to spike protein alone without any virus present. The spike protein alone has been shown to provoke damage to the brain and body ACE 2 receptors are found throughout the body in the endothelial lining of blood vessels, GI system, kidney, skin, heart, on immune cells (platelets, RBC’s mast cells, monocytes), testis, lung (type I and type II alveolar epithelial cells), nasal, and oral mucosa and nasopharynx, and the brain (astrocytes, pericytes, amygdala, cerebral cortex and brainstem, endothelial cells). Studies have shown that spike protein has been shown to damage and cross the blood-brain barrier (BBB). It’s well established that ACE2 plays a regulatory role in neurodegenerative diseases. The level of ACE was decreased in the cerebrospinal fluid of AD patients, and it was negatively correlated with the level of Aβ. So, if we are blocking ACE 2 from spike protein attachment or with antibodies such as an anti-idiotype antibody binding to the ACE 2 receptor, this may well provoke pathophysiological mechanisms to enhance NDD. Protein misfolding is increased. SARS-CoV-2 spike protein can form amyloid and toxic aggregates that can act as seeds to aggregate many of the misfolded brain proteins and can ultimately lead to neurodegeneration. Amyloid beta (Aβ or Abeta) denotes peptides of 36–43 amino acids that are the main component of the amyloid plaques found in the brains of people with Alzheimer's disease. Amyloid Beta has high affinity to spike protein and ACE 2 receptors; viral infection may affect A beta clearance. Although SARS-CoV-2 was rarely detected in the brain, the increase in the S protein in the circulation after SARS-CoV-2 infection may lead to aberrant Aβ homeostasis and promote neurological dysfunction. Case reports post COVID and in VAERS are showing rising numbers of neurodegenerative disease. Alarmingly, we are also seeing numerous cases of CJD or Creutzfeldt–Jakob disease (CJD), also known as subacute spongiform encephalopathy due to prion disease As of June 2022, there are 350 CJD cases reported in VAERS. See: https://jessicar.substack.com/p/modified-spike-protein-rna-injection?s=w#footnote-2 A preprint French study, authored by the late Dr. Luc Montagnier and his colleagues, suggests that the Pfizer, Moderna, and AstraZeneca vaccines may have contributed to the emergence of a new type of sporadic CJD that is much more aggressive and rapid in progression than the traditional form of the disease. This study identified prions in all variants except the Omicron variant and in all COVID vaccines. A peer-reviewed paper out of Turkey also identified sudden CJD cases appearing after getting any of the experimental Pfizer, Moderna, and AstraZeneca vaccines. This study found the disease is showing up 10x quicker – a single day after vaccination. The theory in this initiation of CJD is that the underlying systemic infection, immune activation, inflammatory cytokines, and a prion subset in the S1 protein essentially jumpstart the prion disease. It has been shown that some viruses can serve as a seed to accelerate the aggregation of brain aggregation-prone proteins that are involved in the development of neurodegeneration. SARS-CoV-2 possesses in addition to the prion subsets in S1, several heparin-binding sites within the spike S1 subunit and with activation in the brain, can promote high levels of aggregation-prone proteins such as Aβ, α-synuclein, tau, and prions Will we see ‘new variants’ of other Neurodegenerative disease, for example, in younger and more aggressive onset of Alzheimer’s, Parkinson’s disease, etc.? Diagram from https://pubs.acs.org/doi/full/10.1021/acschemneuro.0c00676#fig1 Figure 1. Schematic representation showing the possible mechanisms that SARS-CoV-2 might involve in the brain’s fibril formation. (A) Structure of SARS-CoV-2, including viral spikes. (B) Aggregation of misfolded proteins on the surface of the viral spike. (C) Cleavage of a viral spike protein by proteases leads to a viral peptide release that possesses high aggregation propensity. This peptide forms toxic fibrils in the brain. In this study, findings showed just what we have been worried about, i.e. how spike protein can induce protein misfolding with the outcome of toxic proteins like tau, alpha synuclein, prion, and amyloid. Credit: https://www.sciencedirect.com/science/article/pii/S0014579301024863 SARS-CoV-2 spike protein interactions with amyloidogenic proteins: potential clues to neurodegeneration.15 Continuing research has identified, as scientists seek to better understand the evolving health situation, other mechanisms of concern including:
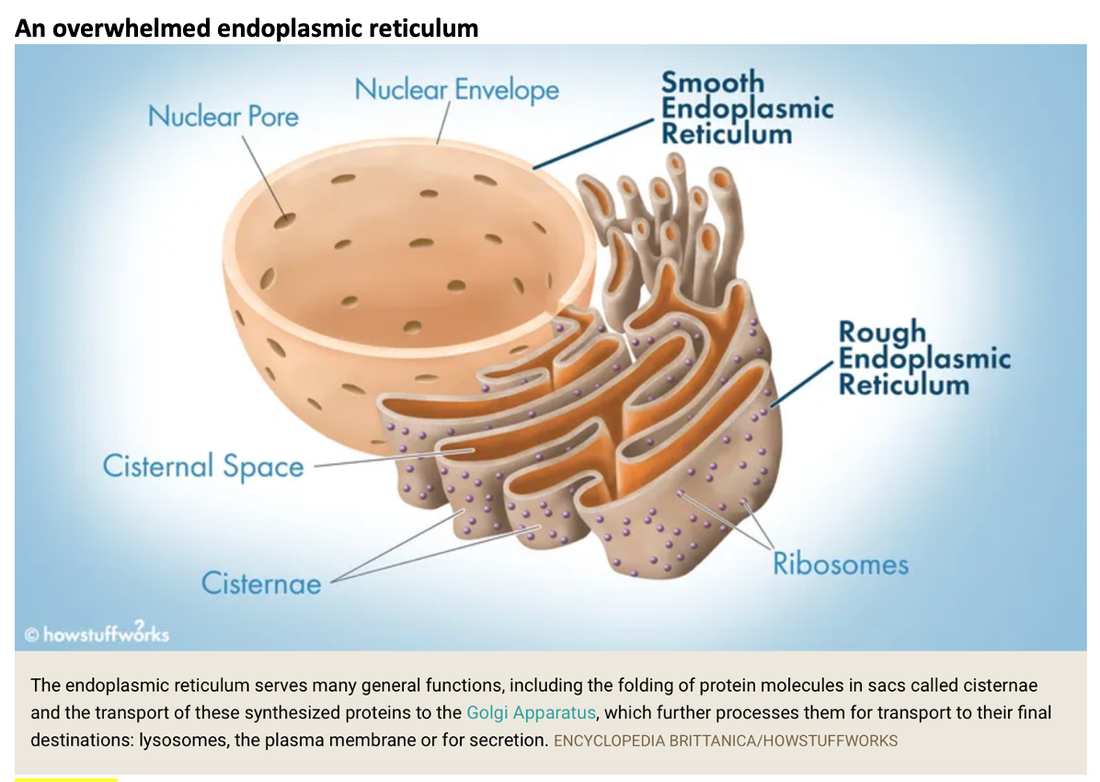
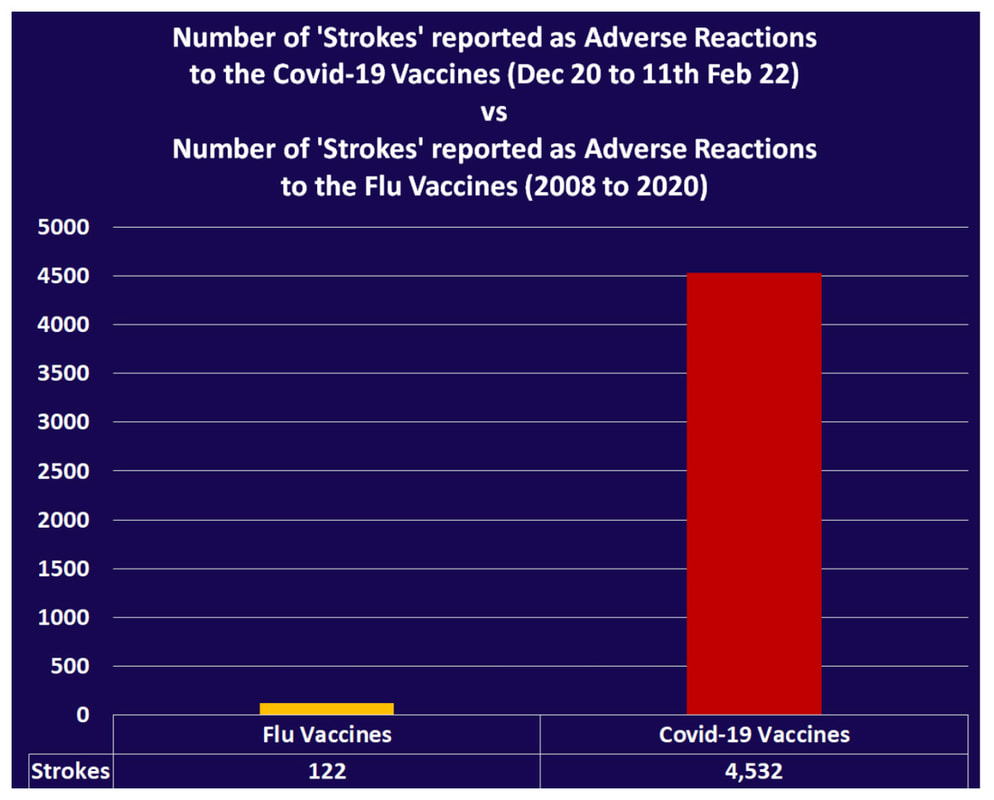
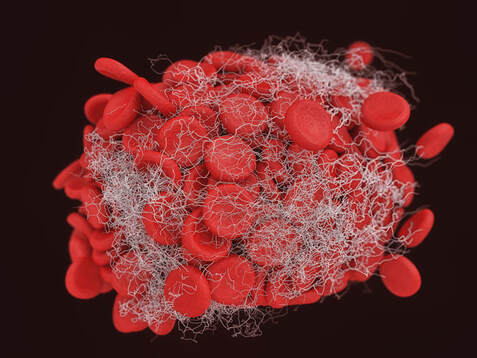
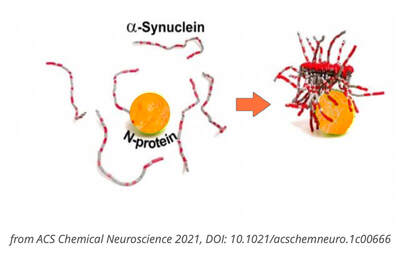
In this study, COVID vaccinations seem to cause a specific amyloid-beta immune-reactivity noted on some PET and CT scans. CREDIT: Jesslyn Shields "What Does the Endoplasmic Reticulum Do?" 24 June 2020. HowStuffWorks.com. <https://science.howstuffworks.com/life/cellular-microscopic/endoplasmic-reticulum.htm> 21 August 2022 The endoplasmic reticulum, found in eukaryotic cells, is a network of tubes or flat sacs, much like a labyrinth of membranes that serves as the factory of the cell, manufacturing and packaging up proteins and lipids to send around the cell, and even outside of it. SARS COV 2 increases protein synthesis that can overwhelm the endoplasmic reticulum (ER) folding capacity, which may result in unfolded protein accumulation source. Spike protein alone does the same thing. Persistent endoplasmic reticulum (ER) stress is thought to drive the pathology of many chronic disorders due to its potential to elicit aberrant inflammatory signaling and facilitate cell death. In neurodegenerative diseases, the accumulation of misfolded proteins and concomitant induction of ER stress in neurons contributes to neuronal dysfunction. Take down of immunity. The innate immune system is the first line of the host defense program against pathogens and harmful substances. Coronaviruses like SARS-CoV-2 have evolved multiple means to evade host antiviral immune responses affecting the innate and adaptive immune systems. In this preprint study in medRxiv, “The BNT162b2 mRNA vaccine against SARS-CoV-2 reprograms both adaptive and innate immune responses,” found that the mRNA BNT162b2 vaccine induces complex functional reprogramming of innate immune responses. There is a direct and well-established link between the innate immunological systems in neurodegenerative and psychiatric diseases. So, will this alteration in our innate immune system pave the way for rising rates of NDD? Immune dysregulation is also behind long COVID. A recent analysis by researchers at the University of New South Wales’s Kirby institute and St. Vincent’s Hospital Sydney found in long Covid patients evidence of sustained inflammation and activation of the immune response for at least 8 months after initial infection. Autoantibodies and Molecular Mimicry. Over the course of the past year, research groups have detected unusually high levels of autoantibodies, which can attack the body’s own cells and tissues, in people after a SARS-CoV-2 infection. In Nature in May 2021, immunologists Aaron Ring and Akiko Iwasaki at Yale School of Medicine and their colleagues reported finding autoantibodies in acute COVID-19 patients that target the immune system and brain; they are now investigating how long the autoantibodies persist and whether they can damage tissues. This month, Cedars-Sinai Medical Center cardiologist Susan Cheng and protein chemist Justyna Fert-Bober wrote in the Journal of Translational Medicine that autoantibodies could last up to 6 months after infection, although the researchers did not correlate autoantibodies’ persistence with ongoing symptoms. Autoantibodies have been implicated in several neurodegenerative diseases. Will we also see an onslaught of autoimmune disease? Exosomes act as a communication network for cells. When a cell is under stress, it releases exosomes containing some of the molecules that are stressing it. Exosomes act as intercellular messengers that give the ability to communicate between both cells of the same type and other cell types. So, in the case of the COVID vaccines, the exosomes contain spike protein and microRNA; microRNAs are signaling molecules that can influence cell function. Two microRNAs, miR-148a and miR-590, are excreted in the exosomes along with the spike protein. This significantly disrupts the type-1 interferon response in any cell, including immune cells. Type-1 interferon keeps latent viruses like herpes and varicella (which causes shingles) viruses in check, so if your interferon pathway is suppressed, these latent viruses can also start to emerge. It is vital to have a healthy innate immune system. Prion spreading and exosomes. Exosomes provide an environment that can induce the conformational conversion of native proteins into aggregates that can be transmitted to otherwise aggregate-free cells in the brain. Circulating exosomes are strongly involved in SARS-CoV-2 infection. But in this study, circulating exosomes with spike protein were noted after immunization. So, will these exosomes provide a transport system for neurological prion disease? Exosomes easily cross the blood brain barrier. The role of prions has been shown in neurodegenerative diseases like Alzheimer’s, Parkinson’s disease, Huntington’s disease, amyotrophic lateral sclerosis (ALS), and multiple sclerosis (MS). In this review the authors provided the latest information about the role of viruses, prions and miRNAs in neurodegeneration and neurodegenerative disorders leading to dementia. It is well known that amyloidogenic proteins or peptides, like Aβ, α-synuclein, tau, and huntingtin, can also spread from cell to cell or can be transmitted from animal to animal or human to animal in a prion-like fashion to cause neurodegenerative disease. The spike protein houses a prion domain; this was designed for higher affinity of the SARS-CoV-2 receptor binding domain (RBD) to the ACE 2 receptor. A study in the Journal of Immunology in November 2021 found that the spike proteins induced by the Pfizer-BioNTech COVID‑19 vaccine are carried by extracellular vesicles called exosomes and circulate throughout the body Credit: Dr Richard Fleming Other concerns about spike protein. Vascular Damage A study at bioRxiv on December 2021 showed that the SARS‑CoV‑2 spike protein by itself promoted vascular inflammation and platelet activation, which the authors suggested was “a pathogenic mechanism to explain thrombosis associated with COVID‑19” SARS-CoV-2 spike protein have the potential to cause microvascular injury to the brain, heart, liver, and kidneys. The association between COVID-19 and blood clots was recognized early in the pandemic among hospitalized COVID-19 patients. Two studies published by JAMA Cardiology in 2021 discuss adverse effects associated with COVID-19 vaccines; one investigation describes vaccine-induced immune thrombotic thrombocytopenia with cerebral venous sinus thrombosis (VITT with CVST) linked to the AstraZeneca/Oxford and Johnson & Johnson vaccines. But the mRNA vaccines can cause stroke and vascular events as well. There are numerous reports of stroke, venous thrombosis, blood clots, myocardial infarction and other information that has been submitted to VAERS. Another study examined the cases of 15 adolescents who experienced short-term myocarditis after receiving the Pfizer/BioNTech vaccine. Multiple peer-reviewed articles now express the same concerns of myocardial injury after the COVID vaccines. A paper published in Nature shows rates of myocarditis post vaccine that may be up to 140 times the normal rate of occurrence. There is concern of subclinical damage to the heart as well. This study, suggests 1 in 43 vaccinated children could have subclinical heart damage.  A retrospective population-based study that analyzed call data from Israel’s National Emergency Medical Services (IEMS) found a more than 25% increase in calls for both cardiac arrest and acute coronary syndrome among 16–39-year-olds during the first 5 months of 2021, as compared to the years 2019-2020. They found that the emergency calls were “significantly associated” with the rates of 1st and 2nd vaccine doses administered to this age group, but not with COVID-19 infection rates. Microclotting Some researchers are looking at another possible culprit for long COVID: tiny clots in the blood. In an acute SARS-CoV-2 infection, small clots can form that can damage cells that line blood vessels. Resia Pretorius, a physiologist at Stellenbosch University in South Africa, and her colleagues published preliminary evidence in August in Cardiovascular Diabetology that microscopic clots can linger after an infection clears. Data is accumulating that this same phenomenon can happen in some patients after the COVID vaccination. They use fluorescence microscopy to report large amyloid microclots and hyperactivated platelets present in blood samples from Long COVID/PASC and showed that these deposits are highly resistant to fibrinolysis. They also report also report genetic results in nine patients based on the knowledge that oxidative stress and platelet activation may partly be caused by the deleterious effect of folate deficiency on MTHFR activity and homocysteine levels. Their studies suggest amyloid form as a prion that is highly resistant to proteolysis. D dimer is a marker of fibrinolytic activity and is commonly elevated in COVID and sometimes in vaccine injured. The soluble spike variants may induce thrombotic events via an antibody-mediated mechanism when binding to ACE2-expressing endothelial cells in blood vessels. Other possible causes of thrombosis include spike protein interactions with different C-type lectin receptors, heparin sulfate proteoglycans, and the CD147 receptor. Platelets also become activated by spike protein for they carry ACE 2 receptors. Binding of the spike protein to platelets can also cause the platelets to become activated; activated platelets tend to clump, which can lead to the formation of clots. Activated platelets express amyloid which as we have learned can have a deleterious effect on the brain and the body. Spike proteins binding to the cells that line our blood vessels can cause these cells to over-produce cell-signaling cytokines that can potentially contribute to dangerous cytokine storms, as we have previously discussed. Increased inflammation is linked to increased risk of clotting in a perpetual dance. Various investigators have shown that the endocytosed spike glycoprotein, even in the absence of viral RNA, can induce a caspase-3 mediated cell death, complement activation, which could lead to a hypercoagulable state, and the increase of many cytokines/proteins associated with severe COVID-19, including TNFα, IL6, IL8, IL1β, and p38. Microclotting and vascular disease have been linked to neurodegenerative disease. Viral persistence/viral reactivation. The late-based research found certain viral molecules could enhance the aggregation of toxic proteins linked with several neurodegenerative-diseases. Multiple different viruses can be reactivated after COVID and after the COVID vaccines including varicella, Epstein-Barr virus, hepatitis, herpes, herpes simplex, and cytomegalovirus. Viral infections and inflammation subsequently may prime neurons and immune cells in the brain, rendering neuronal populations vulnerable to degeneration in the face of subsequent insults. The innate immune response is an early line of defense against microbes (bacteria or viruses) that is launched in the first hours of infection. Viruses can induce brain dysfunction by either direct cytolytic effects or bystander inflammatory reactions - and viral and microbial infections are a risk factor for NDD. Lingering mRNA In this study, it was found that exosomes carry mRNA post vaccine; Could retained mRNA remain viable to continue the message to create more spike proteins? Other studies have shown mRNA lingering in lymph nodes X 2 months (Roitgen, et al Cell) Other Mechanisms Credit: Creative Commons Advanced Senescence At its core, the movie, Curious Case of Benjamin Button is about how we live, given the inexorable reality of death. Whether we live forward into old age or backward into infancy, the result is the same: We die. Benjamin Button aged backwards, but his mind did not Senescence as a central hallmark of aging and constitutes a stress response triggered by insults associated with aging such as genomic instability and telomere attrition, which are primary aging hallmarks themselves. Aging and senescence are associated with a risk of NDD. And numerous studies suggest S1 drives senescence. SARS-CoV-2 spike protein expressing epithelial cells promotes senescence associated secretory phenotype in endothelial cells and increased inflammatory response. We know too that spike protein influences the mitochondria. Mitochondrial dysfunction and downstream effects of increased oxidative stress and impaired autophagy will drive neurodegeneration. The coming wave is beginning to crest. As I have previously discussed, the UK Biobank studies indicated a loss of cortical brain volume following a COVID infection (see https://www.suzannegazdamd.com/blog---long-covid/long-covid-and-the-brain-what-happens-when-the-storm-passes). And in an October 2021 publication in JAMA Network Open, scientists reported on a data analysis of a cohort study of 740 patients at Mount Sinai Health System during the period of April 2020 to May 2021. They noted “a relatively high frequency of cognitive impairment several months after patients contracted COVID-19.” One published study linked biomarkers indicative of Alzheimer's disease and dementia to those found in recovered COVID patients who experienced cognitive impairment. Now in another new study, published in JAMA Network Open, researchers in Norway found that 12 percent of patients reported continual issues with concentration eight months after they had Covid-19. Eleven percent had ongoing memory problems, leaving clinicians to ponder just how long these effects could last? A study from researchers at Columbia University Vagelos College of Physicians and Surgeons reports that the brains of a small sample of patients who died of COVID display some of the same molecular changes found in the brains of people with Alzheimer’s disease. The Columbia researchers found high levels of phosphorylated tau in the brains of the COVID patients in addition to defective ryanodine receptors. Their findings link the inflammatory response to SARS-CoV-2 infection with the neuropathological pathways causing tau hyperphosphorylation typically associated with Alzheimer's. Movement Disorders An additional study examined post-COVID symptoms in a small number of Parkinson’s patients. Among the symptoms that persisted after COVID infection were worsening of motor function, increased levodopa daily dose requirements, fatigue, cognitive disturbances, and sleep disturbances. Experts believe that in people with Parkinson’s, the molecules of a protein in the brain called alpha-synuclein clump together at the cellular level to form toxic amyloid fibrils. Over time, these kill off the neurons that produce the neurotransmitter dopamine, which is essential for motor control. For more about alpha-synuclein see: https://www.suzannegazdamd.com/scientifically-speaking1/alpha-synuclein-in-neurodegenerative-disease Scientists at the University of Twente in Enschede, the Netherlands, have now found evidence that one of the proteins in SARS-CoV-2 may speed up the conversion of alpha-synuclein to amyloid fibrils. Their study, which involved experiments in test tubes using human cell cultures, appears in ACS Chemical Neuroscience. In a preprint study published at medRxiv.com, researchers aimed to identify a potentially important cluster of similar patient-reported symptoms from individuals diagnosed with post-acute sequelae SARS-CoV-2 infection (PASC). The results showed that patients experiencing PASC exhibit a prolonged and debilitating symptom complex that prominently involves vibrations and tremors. These are also common symptoms that have occurred in some instances following vaccination. Where do we go from here? I have reviewed various mechanisms that could trigger a tsunami of neurodegenerative disease, but have only scratched the surface of this complex topic and the potential implications for our brain health. I remained gravely concerned as to what the future may hold given the unprecedented increase in neurological problems that I have already seen in my own practice of 35 years, as well as in the studies we will continue to closely follow. While we do not as yet know what lies ahead, the need for ongoing and more in-depth research is clear. We are fortunate in that so many brilliant clinicians are continuing to provide additional insight regarding these ever-evolving events – so we remain hopeful that with more study, more investigations, and more clarity that we will have the answers we all need to best support our patients’ health. As always, please reach out to our offices if you have questions or would like to schedule a visit. In hope and healing, Dr. Suzanne Gazda Reference sources and additional articles: Manalac, T. Long COVID Evidence Begins to Mount, Suggesting Biomarkers and a "Triangle" Effect. Biospace. July 11, 2022. https://www.biospace.com/article/boston-researchers-find-blood-biomarker-for-long-covid/ Swank, Z., Senussi, Y., Alter, G., Walt, D.R. Persistent circulating SARS-CoV-2 spike is associated with post-acute COVID-19 sequelae. medRxiv. June 14, 2022. https://www.medrxiv.org/content/10.1101/2022.06.14.22276401v1.full.pdf Röltgen, K., Nielsen, S.C.A, Silva, O., et al. Immune imprinting, breadth of variant recognition, and germinal center response in human SARS-CoV-2 infection and vaccination. Cell Volume 185, Issue 6, 2022, Pages 1025-1040.e14, ISSN 0092-8674. https://doi.org/10.1016/j.cell.2022.01.018 Hsu JT, Tien CF, Yu GY, et al. The Effects of Aβ1-42 Binding to the SARS-CoV-2 Spike Protein S1 Subunit and Angiotensin-Converting Enzyme 2. Int J Mol Sci. 2021;22(15):8226. Published 2021 Jul 30. doi:10.3390/ijms22158226 https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8347908/ Sofie Nyström and Per Hammarström. Amyloidogenesis of SARS-CoV-2 Spike Protein. Journal of the American Chemical Society. 2022 144 (20), 8945-8950 DOI: 10.1021/jacs.2c03925. https://pubs.acs.org/doi/full/10.1021/jacs.2c03925?source=cen Livia Rosa-Fernandes, Lucas C. Lazari, Janaina Macedo da Silva, Vinicius de Morais Gomes, Rafael Rahal Guaragna et al. SARS-CoV-2 activates ER stress and Unfolded protein response bioRxiv 2021.06.21.449284; doi: https://doi.org/10.1101/2021.06.21.449284 Sprenkle, N.T., Sims, S.G., Sánchez, C.L. et al. Endoplasmic reticulum stress and inflammation in the central nervous system. Mol Neurodegeneration 12, 42 (2017). https://doi.org/10.1186/s13024-017-0183-y https://molecularneurodegeneration.biomedcentral.com/articles/10.1186/s13024-017-0183-y B Balakrishnan, K Lai. Modulation of SARS-CoV-2 Spike-induced Unfolded Protein Response (UPR) in HEK293T cells by selected small chemical molecules bioRxiv 2021.02.04.429769; doi: https://doi.org/10.1101/2021.02.04.429769 Versteeg GA, van de Nes PS, Bredenbeek PJ, Spaan WJ. The coronavirus spike protein induces endoplasmic reticulum stress and upregulation of intracellular chemokine mRNA concentrations. J Virol. 2007;81(20):10981-10990. doi:10.1128/JVI.01033-07 Ryan, F.J., Hope, C.M., Masavuli, M.G. et al. Long-term perturbation of the peripheral immune system months after SARS-CoV-2 infection. BMC Med 20, 26 (2022). https://doi.org/10.1186/s12916-021-02228-6 Bansal S, Perincheri S, Fleming T, et al. Cutting Edge: Circulating Exosomes with COVID Spike Protein Are Induced by BNT162b2 (Pfizer-BioNTech) Vaccination prior to Development of Antibodies: A Novel Mechanism for Immune Activation by mRNA Vaccines. J Immunol. 2021;207(10):2405-2410. doi:10.4049/jimmunol.2100637 Interaction between SARS-CoV-2 N-protein and α-synuclein accelerate amyloid formation https://21a86421-c3e0-461b-83c2-cfe4628dfadc.filesusr.com/ugd/659775_c3b0844fc2794d71bbe094b06eda0478.pdf The Potential Role of SARS-COV-2 in the Pathogenesis of Parkinson's Disease https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7527541/ COVID Protein Interacts With Parkinson’s Protein, Promotes Amyloid Formation https://neurosciencenews.com/covid-parkinsons-19795/ Young MJ, O'Hare M, Matiello M, Schmahmann JD. Creutzfeldt-Jakob disease in a man with COVID-19: SARS-CoV-2-accelerated neurodegeneration? Brain Behav Immun. 2020;89:601-603. doi:10.1016/j.bbi.2020.07.007 COVID vaccine and the risk of prion disease https://scivisionpub.com/pdfs/covid19-rna-based-vaccines-and-the-risk-of-prion-disease-1503.pdf Additional studies related to microvascular injury and thrombosis: Endothelial cell damage is the central part of COVID-19 and a mouse model induced by injection of the S1 subunit of the spike protein https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7758180/ Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: A report of five cases https://pubmed.ncbi.nlm.nih.gov/32299776/ ARS-CoV-2 Spike Protein Impairs Endothelial Function via Downregulation of ACE 2 https://www.ahajournals.org/doi/full/10.1161/CIRCRESAHA.121.318902 Inflammatory microclots in blood of individuals suffering from Long COVID https://www.sciencedaily.com/releases/2021/10/211004104134.htm Kuvandik, A. Creutzfeldt-Jakob Disease After the COVID-19 Vaccination. Intensive Care 12-2021. https://cms.galenos.com.tr/Uploads/Article_50671/TYBD-0-0.pdf COVID-19 vaccine-induced myocarditis: Case report with literature review. https://www.sciencedirect.com/science/article/pii/S1871402121002253?via%3Dihub Myopericarditis after vaccination, Vaccine Adverse Event Reporting System (VAERS), 1990–2018 https://www.sciencedirect.com/science/article/pii/S0264410X20316145?via%3Dihub Montgomery J, Ryan M, Engler R, et al. Myocarditis Following Immunization With mRNA COVID-19 Vaccines in Members of the US Military. JAMA Cardiol. 2021;6(10):1202–1206. doi:10.1001/jamacardio.2021.2833 143 Case Reports of Vaccine Related Myo/Pericarditis & Other Cardiac Injuries Excluding Thrombotic Injuries with Case Report Excerpts https://ashmedai.substack.com/p/144-case-reports-of-vaccine-related?s=r A.L. Goss, R.D. Samudralwar, R.R. Das, et al. ANA Investigates: neurological complications of COVID-19 vaccines. Ann Neurol, 89 (2021), pp. 856-857 Manuela De Michele, Joshua Kahan, Irene Berto et al. Cerebrovascular Complications of COVID-19 and COVID-19 Vaccination. Circulation Research. 2022;130:1187–1203 https://doi.org/10.1161/CIRCRESAHA.122.319954 Karlstad Ø, Hovi P, Husby A, et al. SARS-CoV-2 Vaccination and Myocarditis in a Nordic Cohort Study of 23 Million Residents. JAMA Cardiol. 2022;7(6):600-612. doi:10.1001/jamacardio.2022.0583 Larson KF, Ammirati E, Adler ED, et al. Myocarditis After BNT162b2 and mRNA-1273 Vaccination. Circulation. 2021;144(6):506-508. doi:10.1161/CIRCULATIONAHA.121.055913 Walach H, Klement RJ, Aukema W. The risk-benefit ratio of Covid-19 vaccines: Publication policy by retraction does nothing to improve it. Clin Transl Discov. 2022;2(1):e35. doi:10.1002/ctd2.35 Patone M, Mei XW, Handunnetthi L, et al. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat Med. 2022;28(2):410-422. doi:10.1038/s41591-021-01630-0 Additional studies related to senescence Meyer K, Patra T, Vijayamahantesh, Ray R. SARS-CoV-2 Spike Protein Induces Paracrine Senescence and Leukocyte Adhesion in Endothelial Cells. J Virol. 2021;95(17):e0079421. doi:10.1128/JVI.00794-21 Kritsilis M, V Rizou S, Koutsoudaki PN, Evangelou K, Gorgoulis VG, Papadopoulos D. Ageing, Cellular Senescence and Neurodegenerative Disease. Int J Mol Sci. 2018;19(10):2937. Published 2018 Sep 27. doi:10.3390/ijms19102937 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6213570/ Advanced senescence will drive neurodegenerative disease https://www.biorxiv.org/content/10.1101/2021.04.16.440215v1.full SARS-CoV-2 Spike Protein Induces Paracrine Senescence and Leukocyte Adhesion in Endothelial Cells https://journals.asm.org/doi/10.1128/JVI.00794-21 SARS-CoV-2 causes senescence in human cells and exacerbates the senescence-associated secretory phenotype through TLR-3 https://www.aging-us.com/article/203560/text Senolytics reduce coronavirus-related mortality in old mice. https://www.science.org/doi/10.1126/science.abe4832
3 Comments
Gabriela Nuñez
10/1/2022 05:24:20 pm
Thank you! Very informative and well argued.
Reply
Raymand Ail
11/24/2022 02:39:44 pm
My heart is full of Joy. i don't know how to thank you Lord for what you have use Dr Abiola Efe to do in my life. All thanks to God Almighty who use Dr Abiola Efe to cure me from Herpes virus with his herbal Medicine, For those suffering from the same Virus You can call Or WhatsApp Dr Abiola Efe on whatsApp [+2348100609098 ] He can also cure your health issues Like Cancer, HBP, Hepatitis B Virus, Herpes child bearing and Pile Infection et
Reply
Catherine Roberta
12/30/2022 04:49:37 am
I was diagnosed 2011. i was diagnosed with Parkinson’s disease. My symptoms progressed quickly. Soon i was having difficulty breathing, swallowing and even walking short distances. With the help of Health Herbs Clinic natural herbs I have been able to reverse my symptoms using diet, herbs, which i feel has made the most difference. The Parkinson’s natural formula immensely helped my condition, it reversed my Parkinson’s. my slurred speech, then the tremors, and mobility gradually disappeared, even my handwriting is getting better visit their website at healthherbsclinic . com people are suffering from this horrible disease due to lack of information.
Reply
Your comment will be posted after it is approved.
Leave a Reply. |
AuthorSuzanne Gazda M.D. Neurologist Archives
January 2024
Categories |



 RSS Feed
RSS Feed