|
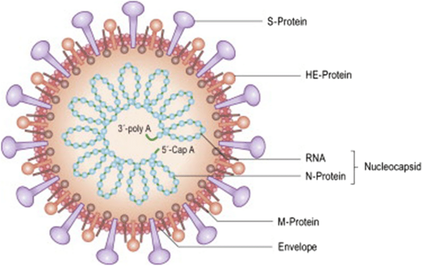
While we certainly know more about COVID 19 than we may have when this pandemic first began over two years ago, there is much still to be learned as events continue to evolve and new information comes to light. One big question remains – what effects may linger for weeks, or even months and longer, after someone has been infected with the virus? And what could this mean as far as our brain health? Studies to date already have shown that in some patients there are ongoing neurological and other issues that may not immediately resolve and comprise some of the symptoms associated with long COVID or long-haulers syndrome. Learn more about the lingering effects of long COVID at: https://www.suzannegazdamd.com/blog---long-covid/the-diverse-neurological-and-other-symptoms-of-long-covid What is COVID 19? We know that COVID 19 is caused by a coronavirus called SARS-CoV-2 a mRNA virus that is thought to have originated in Wuhan, China in or around December 2019. Highly contagious and communicable through respiratory droplets, it quickly spread to virtually all points of the globe. It is part of the coronavirus family, which includes common viruses that cause a variety of diseases from simple colds to more complex illnesses such as severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS). However, the world has never witnessed anything like this present virus and its iterations. To date, there have been over 900,000 deaths in the United States alone and of the millions of infections it is estimated that upwards of 35% of all diagnosed cases will go on to develop long COVID, also known as post-acute COVID 19 syndrome (PACS).1 Especially concerning for patients and clinicians alike is that the likelihood of developing long COVID appears to be unrelated to the severity of the original infection - and even when the patient no longer tests positive for the virus or antibodies. For additional information, see the first blog in our series, “What is long COVID?” at: https://www.suzannegazdamd.com/blog---long-covid/what-is-long-covid More about PASC or long-haul COVID. In PASC (post-acute sequelae SARS-CoV-2 infection), there is an activation of the immune system that persists for months, which can affect neural connections in the brain. The role of cytokine storms, which occur when the overproduction of cytokines sets off a positive feedback loop of immune responses, in aggravating COVID infections was known early in the pandemic. What was not realized in the beginning of all these events is how truly lethal spike protein can be on its own. In the material that follows, I will describe how numerous researchers have yet to find any evidence of retained whole viral genome and suggest to you that what we are dealing with are multiple factors that lead to the development of long COVID. For a detailed discussion about why long COVID occurs, see our previous article in this series: https://www.suzannegazdamd.com/blog---long-covid/why-does-long-covid-happen The next pandemic: long COVID. Studies have found that up to 84% of COVID-19 patients suffer from neurological symptoms, anosmia (loss of sense of taste or smell), epileptic seizures, strokes, loss of consciousness, fatigue, brain fog, and confusion, and other impacts.2 Fortunately, most of these acute neurological symptoms resolve, but in approximately one-third of COVID patients, long-term symptoms remain. Brain fog, headaches, and cognitive dysfunction are some of the most common ongoing neurological symptoms. One international study of individuals with long COVID documented 203 different symptoms across ten body systems. More than 88% of the 3,762 people who completed the online survey reported memory problems and cognitive dysfunction, making these the “most persistent and pervasive symptoms in this cohort, equally common across all age groups.”3 What is happening in the brains of long haulers?
But some studies have shown that the virus has difficulty getting past the brain’s defense system, the blood–brain barrier (BBB), and that it doesn’t necessarily attack neurons in any significant way. Other investigations looking for residual whole virus have found no evidence of this, and others, including a renowned researcher at the National Institutes of Health, Dr. Avindra Nath, have stated that while most post-COVID autopsies primarily show just “viral fragments,” there is no presence of an ongoing viral infection. Dr Nath’s findings. 30 brain samples from COVID 19 patients ages 5 to 80 were studied; all had died either at home or in a long-term care facility. The samples were analyzed using the strongest magnetic resonance imaging (MRI) available for use on the human body. These images provided extraordinary detail of brains affected by the SARS-CoV-2 virus. Using advanced technology and careful investigations, high levels of neuroinflammation and microvascular changes were found, but Nath's team was unable to identify active virus in the brain tissue samples.4 Dr. Nath and his team’s findings showed no whole virus, but did indicate widespread inflammation in the brain. With COVID-19, there is an activation of the immune system that persists for months, which can affect neural connections in the brain. Many believe that the viral fragments, especially spike protein, may be wreaking havoc long after the acute infection has cleared. Microclotting and vascular injury in long COVID. Pathology and autopsy studies have found particles of SARS-CoV-2 inside endothelial cells, and a growing body of evidence suggests that the endothelium plays a key role in the disease.5 Normally the endothelial lining is like Teflon and nothing sticks to it (Endothelial cells are the main type of cell found in the inside lining of blood vessels, lymph vessels, and the heart) Investigators also identified the presence of fibrinogen, a protein which normally plays a role in blood clotting. Vascular injury is occurring in COVID with congested blood vessels, microhemorrhages, white hyperintensities all indicate the blood vessels are affected and the brainstem may be a particular target. SARS-CoV-2 spike protein S1 induces fibrinogen that are resistant to fibrinolysis implications for microclot formation in COVID 19. Fibrinolysis is a process that prevents blood clots from growing and becoming problematic. Since early 2020, researchers have pointed out that acute COVID 19 is not only a lung disease, but also significantly affects the vascular (blood flow) and coagulation (blood clotting) systems. A recent study in Dr. Resia Pretorius’ lab in South Africa revealed that there is significant microclot formation in the blood of both acute Covid-19 and long Covid patients. They found high levels of various inflammatory molecules trapped in the persistent microclots, including clotting proteins like plasminogen, fibrinogen and Von Willebrand factor (VWF), and also Alpha-2 antiplasmin (a molecule that prevents the breakdown of microclots). The presence of persistent microclots and hyperactivated platelets (also involved in clotting) perpetuates coagulation and vascular pathology, resulting in cells not getting enough oxygen in the tissues to sustain bodily functions (known as cellular hypoxia). Widespread hypoxia may be central to the numerous reported debilitating symptoms.6 Additional findings in a recent preprint publication outlines details of a cohort of 845 people with long COVID. This study looked at treatment with antiplatelet therapy in patients with Long Covid found to have evidence of microclotting. Clearly there is an urgent need to further explore this area for the most comprehensive options. In another writing, I have reviewed the work of Dr Bruce Patterson and colleagues, who found retained spike protein in nonclassical monocytes for up to 15 months post COVID – and these cells easily cross the BBB. Immune System Dysregulation. Dr. Nath has also suggested that T-cell exhaustion may be causing the immune system to compensate by increasing the efforts of the early acting, highly inflammatory, innate immune system. It may be necessary to enhance the later-acting adaptive side of the immune system, while knocking down the innate immune system. He and many of his colleagues believe the studies show that it is not the virus that crosses into the brain, but that the neurological impacts result more from inflammation, disruption of the blood-brain barrier (BBB) and micro-clotting, e.g., vascular pathology (and as previously noted, the autopsies he and his team performed showed only viral fragments, but no whole viral genome). Another study showed that inflammatory markers, such as interleukin (IL)-6, tumor necrosis factor (TNF)-α, and C-reactive protein, were elevated in blood plasma from 24 patients six to nine months following infection.7 Researchers at Columbia University say that they found no signs of virus inside the patients' brain cells, but saw many brain abnormalities that could explain the confusion and delirium observed in some patients with severe coronavirus and the lingering "brain fog" in those with mild disease. It is possible too that the microglia may have been activated by inflammatory cytokines. This study included only patients who were severely ill and died; these changes may not be seen in patients with mild illness.8 Rogue Autoantibodies. Another study and subsequent findings also point to immune dysregulation post COVID. Researchers analyzed the blood and cerebrospinal fluid of 11 people critically ill with COVID-19 and exhibiting neurological symptoms. All patients produced autoantibodies capable of binding neurons; antibodies that specifically react with self-antigens are called autoantibodies. These antibodies are generated as a result of the loss of tolerance response against self-antigens and can be pathogenic. The loss of the ability of the immune system to distinguish between self and non-self-antigens is the underlying cause of autoantibody development. Besides autoimmune diseases, autoantibodies can be detected in the serum sample of people suffering from cancer or having severe tissue damage. COVID 19 and the COVID vaccine can produce autoantibodies that trigger self-attacking rogue antibodies. Recent studies also noted four specific risk factors for developing long COVID:
But Dr Patterson’s research has of yet not revealed any well-defined risk factors. High levels of autoantibodies could help explain why IVIG has been helpful in COVID and long COVID for it helps to clear the autoantibodies and resets the immune system. And still more studies… In a 2022 publication, researchers reported finding an antibody signature associated with long-term COVID 19 symptoms. The study initially studied blood samples from a cohort of 175 people diagnosed with SARS-CoV-2 infection and 40 healthy controls. The researchers analyzed the blood antibody levels during infection, then followed 134 of the participants for a year afterward, collecting additional blood samples at 6 and 12 months and documenting their symptoms.9 When the researchers compared antibody levels in the blood samples, they found that patients who developed long COVID tended to have lower levels of IgM at the start of infection and lower levels of an antibody called IgG3 six and 12 months after infection than did infected people who didn’t develop the condition. This study and its findings suggests that in some patients, IVIG may be a beneficial treatment option. Autoimmune chaos and molecular mimicry wreak havoc on the body and brain.
In my previous blog, I also discussed immune cross reactivity that occurs with COVID and COVID proteins; this can additionally occur post vaccine as the body responds in an abnormal way to spike protein. Dr. Bruce Patterson’s work with long haulers. Dr. Bruce Patterson, whose research and protocol is featured in a previous blog in our series, has studied and helped thousands of PASC patients. While Dr. Patterson did not find any evidence of whole viral genome, he did find retained spike protein in nonclassical monocytes up to 15 months post infection and post COVID vaccine. Neuropsychiatric symptoms, brain fog, and cognitive impairment.
There are more reports daily that show patients of COVID-19 may continue to suffer memory loss, even months after contracting the virus. One investigation reports nearly a quarter of individuals who've been infected with the coronavirus have problems retaining information and focusing months after contracting the disease. Researchers, examining 740 patients at the Mount Sinai Health System in New York, found that it is relatively common for people who have previously had COVID to struggle with activities including multitasking.11 Note that the study participants were 18 years or older, spoke English or Spanish, tested positive for SARS-CoV-2 or had serum antibody positivity, and had no prior history of dementia. Impaired cognition associated with COVID-19 appears to have a biological versus psychological basis. Brain fog and cognitive impairments are common complaints in PASC despite the severity of the COVID infection. A recent 2022 study in Annals of Clinical and Translational Neurology examined the cerebrospinal fluid in long haulers. Overall, 77% of participants with cognitive PASC had a CSF abnormality, compared with 0% of cognitive controls (P = .01). CSF abnormalities included elevated protein levels with no other explainable cause in 2 of the 13 subjects with PASC and elevated oligoclonal antibodies were identified in 69% of participants with cognitive PASC, compared with 0% of cognitive controls.12 Startling findings from the United Kingdom.
The results have not yet undergone peer review, though they are available online in preprint form. By May 2021, the Biobank study had identified 401 participants who had tested positive for SARS-CoV-2 and who also had useable pre- and post-COVID 19 scans.13 Most of these participants did not spend time in the hospital, representing a group of people with mild to moderate COVID 19 infection. The researchers matched these individuals to 384 other people who showed no evidence of COVID-19 and similar risk factors for COVID-19 infection. Using fine-grain automated analyses of the brain scan images to pick up changes that would not be visible to the naked eye, the researchers noted that compared to controls, participants who had COVID-19 showed:
Individuals who had COVID 19 also showed a larger cognitive decline on several cognitive function tests.11 No whole virus can be found, but viral fragments like spike protein are toxic to the brain. In a study published Dec.16 in Nature Neuroscience, researchers found that the spike protein, often depicted in scientific illustrations as the ‘red arms’ of the virus, can cross the blood-brain barrier in mice. Dr. Banks and the investigating team noted that the S1 protein in SARS-CoV2 and the gp 120 protein in HIV-1 function similarly. They are glycoproteins, which are proteins that have a great deal of sugars on them, hallmarks of proteins that bind to other receptors. Both these proteins function as the arms and hand for their viruses by essentially grabbing onto other receptors. Both can cross the blood-brain barrier and S1, like gp120, are likely toxic to brain tissues. I have discussed in detail the link to spike protein, which binds to a surface receptor on our cells called ACE2, triggering uptake of the virus particle and eventually membrane fusion; ACE2 is the molecule that may help the coronavirus invade the cells. For more details about spike protein, ACE2 receptors, and mechanisms behind long COVID, see our second blog in the series, “Why does long COVID happen?” at: https://www.suzannegazdamd.com/blog---long-covid/why-does-long-covid-happen More news on the toxicity of spike protein.
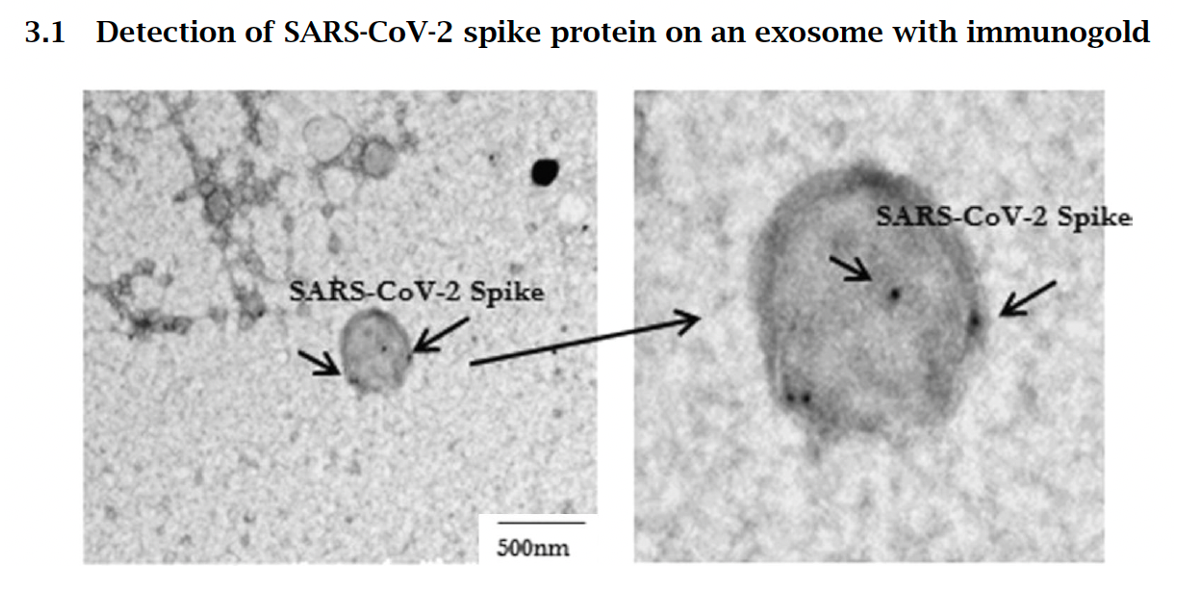
The intense inflammation caused by the COVID-19 infection is called a cytokine storm. The immune system, upon detecting the virus and its proteins, overreacts in its attempt to kill the invading virus. The patient many times is left with brain fog, fatigue and other cognitive issues. Residual components of the virus, such as spike protein cause immune dysregulation, cytokine storm, inflammation of the endothelium, autoantibody production, and other mechanisms that drive brain and systemic symptoms. In this recent study by Bansal et al., the detection of spike protein in persons vaccinated with the Pfizer mRNA vaccine was studied. The most significant finding is that spike protein is found on exosomes, which are cell-derived vesicles, for at least four months after the second injection. This surprisingly long persistence raises the prospect of sustained inflammation within and damage to organs that express the spike protein. And we know that exosomes easily cross the BBB. Going Forward.
Much more research needs to be done so that we can see our way through the fog of long COVID. Certainly, we are learning more every day and open scientific debate is crucial now, perhaps more than ever before. A large bulk of science is research shows that long COVID is not related to an ongoing or residual virus, but instead from components of the virus that have been left as debris for the immune system to basically “clean up.” Part of that debris is spike protein, which in and of itself has been found to be toxic to the body and brain Then there is the looming question being silenced in scientific circles is regarding the COVID vaccinations and their ability to create very high levels of spike protein through genetic modification mechanisms. The vaccines produced against COVID have utilized either mRNA or an adenovirus vector to direct human cells to produce the spike protein against which the body produces mostly neutralizing antibodies. When the COVID-19 vaccines were designed, it was not appreciated that the spike protein could potentially damage cells in the body. With each vaccine injection, billions of mRNA molecules wrapped in a lipid nanoparticle are injected into the body. Given that mRNA has a half-life of around one to two weeks, depending on the mRNA, and during that interim, each mRNA molecule makes around 2,000 to 5,000 spike proteins, this will form trillions of spike proteins. It is for the benefit of all patients and as part of the scientific discovery process that we continue to investigate all mechanisms and processes by which the effects of long COVID or preventative actions could occur in order to identify the most appropriate treatments and medical guidance. In hope and healing for humanity, Dr. Suzanne Gazda References: 1 At Least One Long-Term Symptom Seen in 37% of COVID-19 Patients: Study. Medscape. September 30, 2021. https://www.medscape.com/viewarticle/959924 2 Ross W Paterson, Rachel L Brown, Laura Benjamin, Ross Nortley, Sarah Wiethoff, Tehmina Bharucha, Dipa L Jayaseelan, et al for the UCL Queen Square National Hospital for Neurology and Neurosurgery COVID-19 Study Group, The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings, Brain, Volume 143, Issue 10, October 2020, Pages 3104–3120, https://doi.org/10.1093/brain/awaa240 3 Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. Hannah E. Davis, Gina S. Assaf, Lisa McCorkell, Hannah Wei, Ryan J. Low, Yochai Re'em, et al. The Lancet. (July 15, 2021) https://doi.org/10.1016/j.eclinm.2021.101019 4 Nath, A. et al. Microvascular Injury in the Brains of Patients with Covid-19. New England Journal of Medicine. (2/4/2021). https://www.nejm.org/doi/full/10.1056/NEJMc2033369 5 Peter Libby, Thomas Lüscher, COVID-19 is, in the end, an endothelial disease, European Heart Journal, Volume 41, Issue 32, 21 August 2020, Pages 3038–3044, https://doi.org/10.1093/eurheartj/ehaa623 6 Pretorius, E., Vlok, M., Venter, C. et al. Persistent clotting protein pathology in Long COVID/Post-Acute Sequelae of COVID-19 (PASC) is accompanied by increased levels of antiplasmin. Cardiovasc Diabetol 20, 172 (2021). https://doi.org/10.1186/s12933-021-01359-7 7 Sinead Ahearn Ford, Nonhlanhla Lunjani, Brian McSharry, et al. Long Term Disruption of Cytokine Signalling Networks are Evident Following SARS-CoV-2 Infection. Authorea. April 16, 2021. DOI: 10.22541/au.161860639.93671836/v1 8 Kiran T Thakur, Emily Happy Miller, Michael D Glendinning, Osama Al-Dalahmah, Matei A Banu, Amelia K Boehme, et al. COVID-19 neuropathology at Columbia University Irving Medical Center/New York Presbyterian Hospital, Brain, Volume 144, Issue 9, September 2021, Pages 2696–2708, https://doi.org/10.1093/brain/awab148 9 Cervia, C., Zurbuchen, Y., Taeschler, P. et al. Immunoglobulin signature predicts risk of post-acute COVID-19 syndrome. Nat Commun 13, 446 (2022). https://doi.org/10.1038/s41467-021-27797-1 10 Vojdani A, Vojdani E and Kharrazian D (2021) Reaction of Human Monoclonal Antibodies to SARS-CoV-2 Proteins With Tissue Antigens: Implications for Autoimmune Diseases. Front. Immunol. 11:617089. doi: 10.3389/fimmu.2020.617089 11 Becker JH, Lin JJ, Doernberg M, et al. Assessment of Cognitive Function in Patients After COVID-19 Infection. JAMA Netw Open. 2021;4(10):e2130645. doi:10.1001/jamanetworkopen.2021.30645 12 Apple, A.C., Oddi, A., Peluso, M.J., Asken, B.M., Henrich, T.J., Kelly, J.D., Pleasure, S.J., Deeks, S.G., Allen, I.E., Martin, J.N., Ndhlovu, L.C., Miller, B.L., Stephens, M.L. and Hellmuth, J. (2022), Risk factors and abnormal cerebrospinal fluid associate with cognitive symptoms after mild COVID-19. Ann Clin Transl Neurol. https://doi.org/10.1002/acn3.51498 13 Gwenaëlle Douaud, Soojin Lee, Fidel Alfaro-Almagro, Christoph Arthofer, Chaoyue Wang et al. Brain imaging before and after COVID-19 in UK Biobank. medRxiv 2021.06.11.21258690; doi: https://doi.org/10.1101/2021.06.11.21258690 14 Rhea, E.M., Logsdon, A.F., Hansen, K.M. et al. The S1 protein of SARS-CoV-2 crosses the blood–brain barrier in mice. Nat Neurosci 24, 368–378 (2021). https://doi.org/10.1038/s41593-020-00771-8
0 Comments
Your comment will be posted after it is approved.
Leave a Reply. |
AuthorSuzanne Gazda M.D. Neurologist Archives
January 2024
Categories |



 RSS Feed
RSS Feed