|
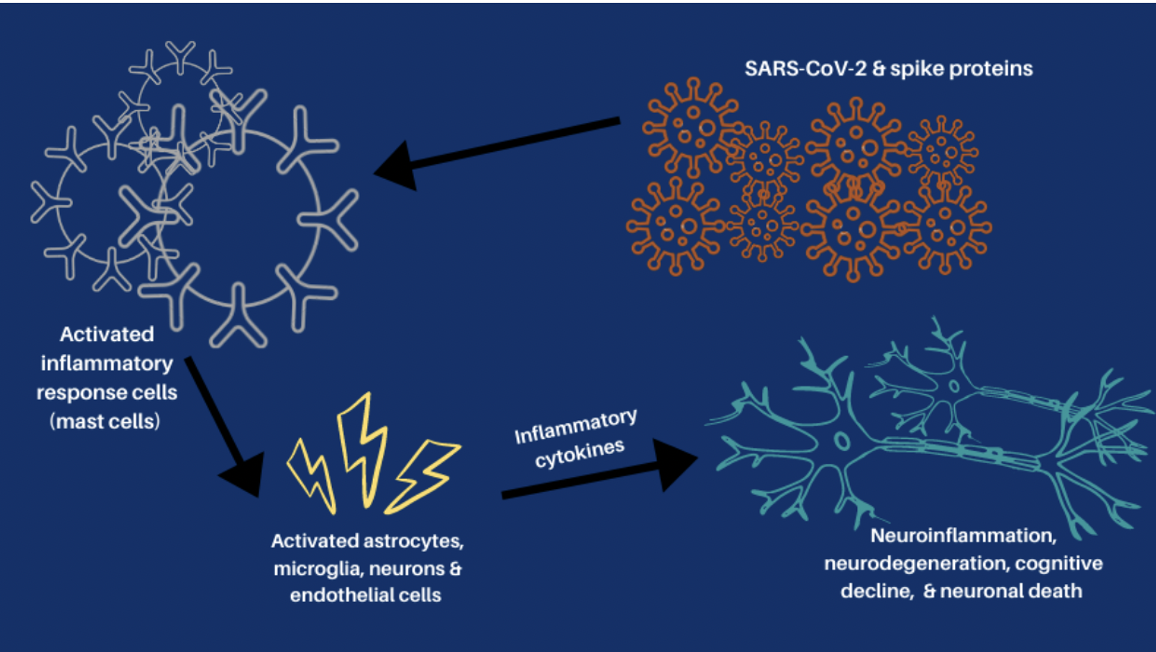
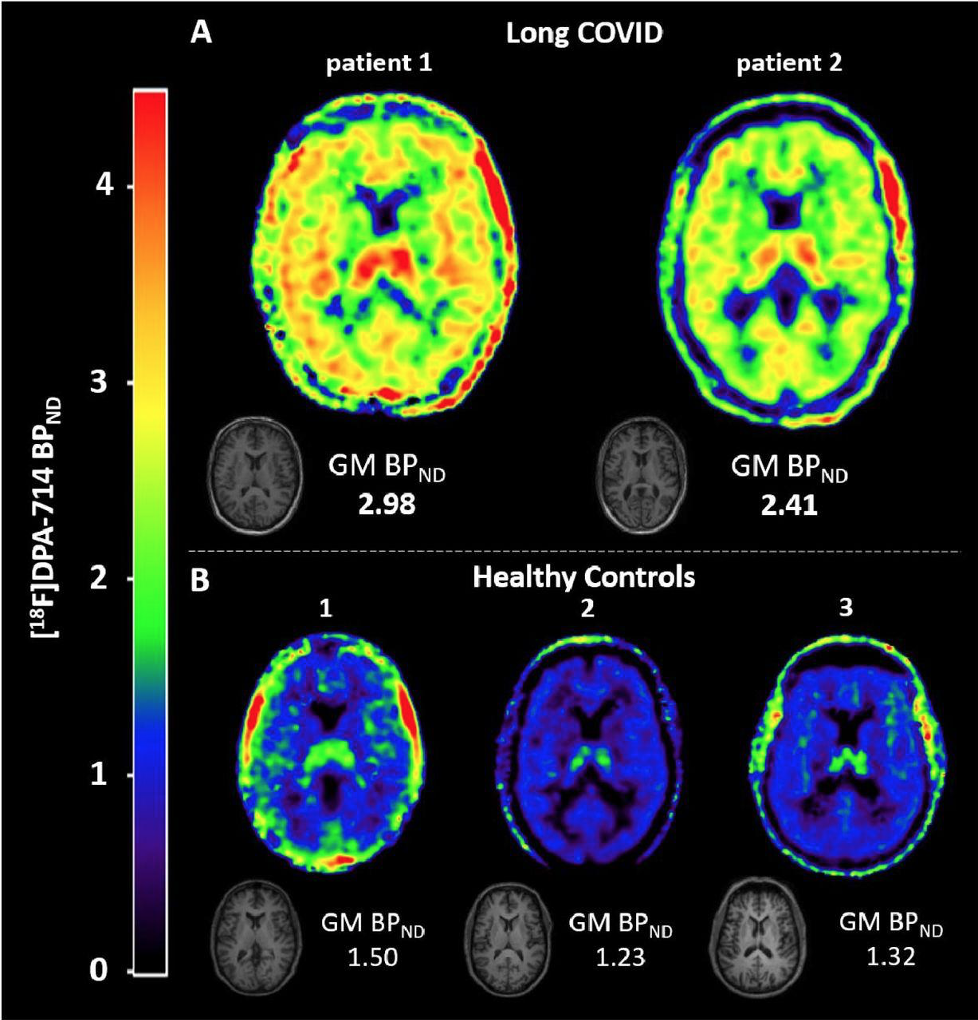
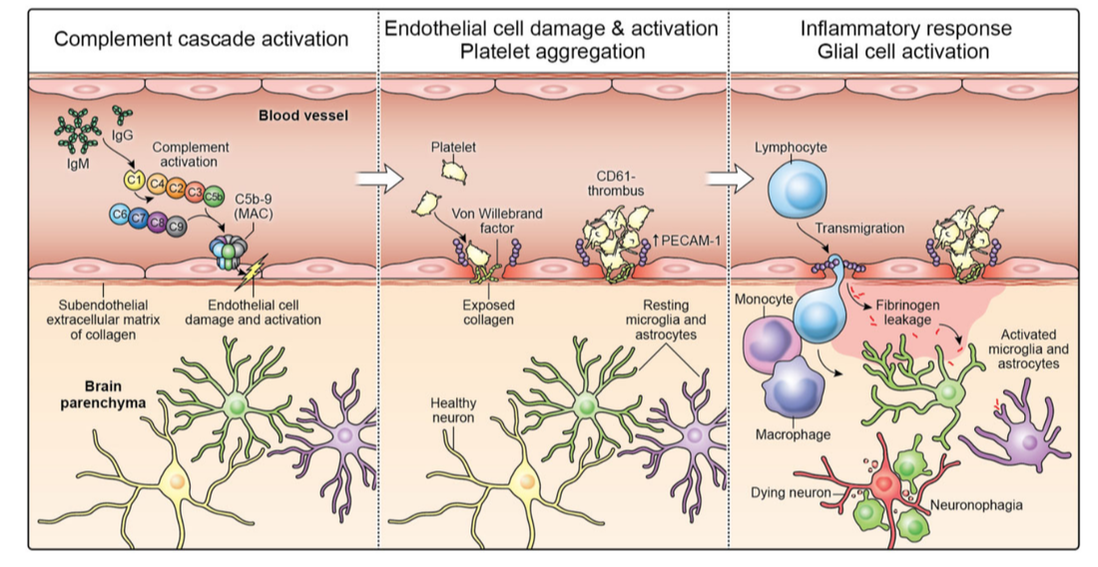
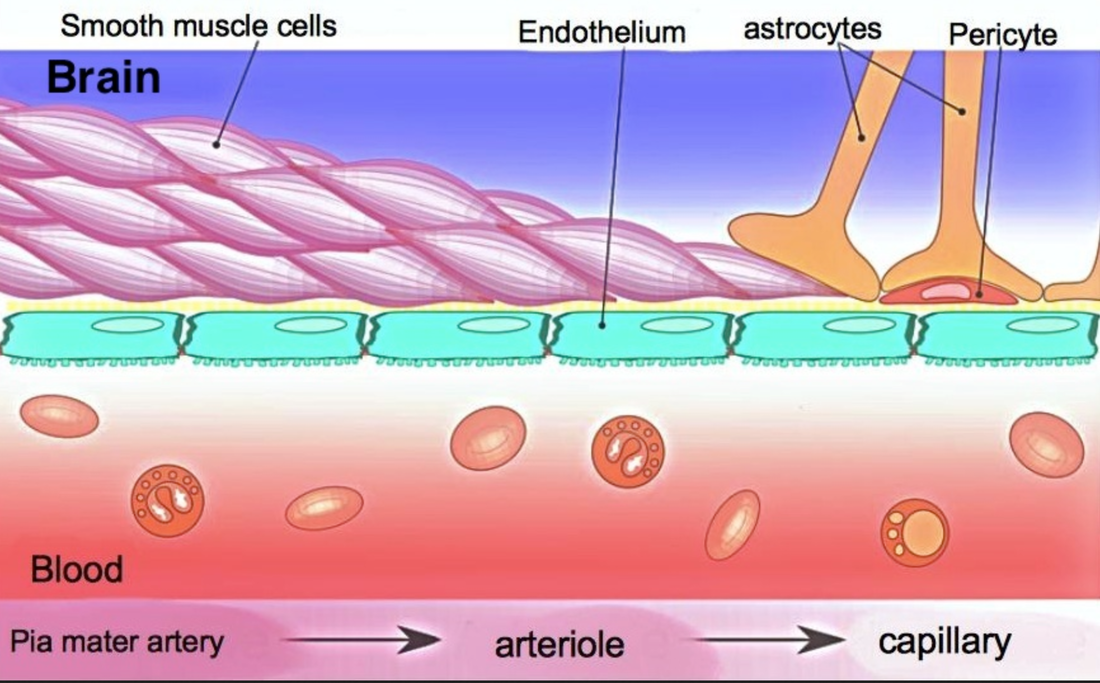
High amounts of inflammation and immune dysregulation: The immune system is on high alert. Credit: Harvard University, The Coronavirus Visualization Team; article Covid Long Haulers: Neurological System; Sabrina Oliveras Immune dysregulation is one of the key mechanisms behind PASC. A recent analysis by researchers at the University of New South Wales’ Kirby institute and St Vincent’s Hospital Sydney found in long Covid patients evidence of sustained inflammation and activation of the immune response for at least 8 months after initial infection. Dr. Bruce Patterson identified key proinflammatory cytokines in long COVID and in a subset of post-vaccine injured patients carrying spike protein in monocytes, about which you can read more at: https://www.suzannegazdamd.com/blog---long-covid/a-protocol-for-treatment-starts-with-tamping-down-inflammation There is a direct interplay between high levels of inflammation and alteration of the clotting mechanisms for inflammatory cytokines are the major mediators involved in coagulation activation and clotting. In a study published in Nature, immune dysregulation was seen up to 8 months following a COVID illness. This sustained inflammatory response following even mild-to-moderate acute COVID-19, was not found following previous coronavirus infection The study, “Long-term perturbation of the peripheral immune system months after SARS-CoV-2 infection,” suggests COVID-19 can have an impact on the immune system many months after the virus is contracted – even among these who have mild symptoms. Additionally, long COVID patients had an immune system in constant high alert, the team reported in January in Nature Immunology. Chronic neuro-inflammation has been linked to memory and other cognitive impairments, as well as Alzheimer’s disease. Answers Found in CSF Credit: CC BY-SA-NC In a new study led by researchers at University of California at San Francisco (UCSF), they discovered a link between people with Long Covid post-recovery brain fog and abnormalities in their spinal fluid. Examinations of the cerebrospinal fluid (CSF) revealed elevated levels of protein, suggesting inflammation, and the presence of unexpected antibodies found in an activated immune system. Some were found in the blood and cerebrospinal fluid, implying a systemic inflammatory response, or were unique to the cerebrospinal fluid, suggesting brain inflammation. Abnormal oligoclonal banding (OCB) patterns were identified in 69% (9/13) of participants with cognitive PASC compared to 0% of cognitive control; OCBs are a sign of inflammation usually from immune activation in the brain. More than 75% of cognitive PASC participants who underwent LP had CSF abnormalities, although they were older than the small group of cognitive controls These findings suggest that post-COVID cognitive symptoms could be due to an increase in inflammation in the brain from underlying immune dysregulation A study published in on the effect of SARS-CoV-2 infection on the brains of primates has revealed parallels with studies carried out on human autopsies. They showed high amounts of neuroinflammation, microhemorrhages and lack of oxygen to the brain in the monkey’s post COVID. Brain impairment seen on PET scans in long COVID. More clues to High Inflammation A brain positron emission tomography (PET) scan is an imaging test of the brain. It uses a radioactive substance called a tracer to look for disease or injury in the brain. From a brain perspective, regional hypometabolism in multiple regions seen on a PET scan likely reflects widespread impairment of neuronal activity. A fluorodeoxyglucose (FDG) PET scan is not only an established biomarker of neuronal function and neuronal injury, but also of CNS inflammation even in the absence of typical MRI findings. Regional glucose consumption can also be correlated with neurotransmission and synapses firing, both of which require a high amount of energy. Multiple studies now show FDG PET hypometabolism could constitute a cerebral biomarker of long COVID. Bottom line….the brain is struggling! A multicenter study looked into brain hypometabolism detected by PET scans done on patients who had had SARS-CoV-2 infection and were referred for suspected neurologic long COVID. Almost half of the scans were interpreted as abnormal, with more than a quarter of them being interpreted as severely affected. As stated by the authors, “This exam could reassure the half of patients for whom a brain impairment was suspected and enable the other half to get access to adequate care, as well as the requisite social and medical recognition for their condition.” In this preprint, long COVID was associated with extensive neuroinflammation seen on PET The inflammations were 76 and 121 percent increased, respectively, as compared to the level seen in the brains of healthy people. Credit: https://www.umcutrecht.nl/en/over-ons/nieuws/infection-and-immunity/jun-6-long-covid-patients-show-extensive-brain-inflammation Autoimmune onslaught on the brain. A recent study, performed at the National Institutes of Health (NIH) was published in the journal Brain. In this study they examined brain autopsies of people who had died from COVID. Rather than detecting evidence of a virus (COVID) in the brain, the team found it was the people's own antibodies that attacked the endothelial cells lining the brain's blood vessels, causing inflammation and damage. In an earlier study from the group, SARS-CoV-2 was not detected in the patients’ brains, also, suggesting the virus was not infecting the brain directly. They found multifocal vascular damage with leakage of serum proteins into the brain parenchyma and widespread endothelial cell activation with platelet aggregates and microthrombi adherent to the endothelial cells along vasculature with immune complement activation. Credit: Brain, Volume 145, Issue 7, July 2022, Pages 2555–2568 Neurovascular injury with complement activation and inflammation in COVID-19 Complement binds to the IgG and IgM antibodies and activates the classic complement pathway. The end product of this cascade, C5b-9 binds to endothelial cells and causes endothelial cell damage. This leads to activation of endothelial cells and clotting factors with increased PECAM-1 and vWF release, resulting in platelet aggregation and thrombus formation. Simultaneously, there is leakage of serum proteins into the perivascular space, which leads to an influx of monocytes and T lymphocytes into the parenchyma. Monocytes differentiate to macrophages and there is activation of microglia and astrocytes in the brain parenchyma. This leads to neuronal injury and neuronophagia. Antibody-mediated cytotoxicity directed against the endothelial cells is the most likely initiating event that leads to vascular leakage, platelet aggregation, neuroinflammation and neuronal injury. Since no virus was found lurking in the halls and chambers in the brain, they conclude that it’s possible the antibodies produced against the SARS-CoV-2 spike protein are what’s triggering this pathology. These antibodies mistakenly target the endothelial cells, which are crucial to the integrity of the blood-brain barrier (BBB). Armin Kübelbeck, CC BY creativecommons.org via Wikimedia Commons Numerous studies have shown that spike protein can damage the endothelial lining by binding to the ACE 2 receptors. These cells then express proteins called adhesion molecules that cause platelets to stick together. High levels of adhesion molecules were found in endothelial cells in the samples of brain tissue in the NIH study. Activation of the endothelial cells brings platelets that stick to the blood vessel walls, causing clots to form and leakage to occur. At the same time the tight junctions between the endothelial cells get disrupted causing the BBB to leak. Once this happens, immune cells such as macrophages may come to repair the damage, setting up inflammation. This, in turn, causes activation of microglia and damage to neurons. In addition to phagocytosing damaged cells, activated microglia secrete inflammatory mediators, including glutamate, quinolinic acid, and TNF-α. Increased quinolinic acid results in higher glutamate and upregulation of NMDA receptors, possibly inducing altered learning, memory, neuroplasticity, hallucinations, and nightmares. Immune dysregulation, molecular mimicry, and rogue autoantibodies. Other studies agree that SARS-CoV-2 does not invade the brain in the majority of cases and so the associated neurological complications post-COVID or vaccine might arise from indirect effects, such as immune activation; although the immune system plays a critical role in controlling the virus, it is immune dysregulation causing pathology in long COVID. In this research from Frontiers of Neurology May 2022, scientists report autoimmune complications to be related to molecular mimicry of SARS-CoV-2 proteins with human proteins. Hexa-peptide and hepta-peptide sharing of SARS-CoV-2 spike glycoprotein with human proteins has been identified as a potential contributor to the occurrence of autoimmune disorders observed in COVID-19 patients. This recent article July 2020 in Viruses again showed spike protein reacting with numerous human tissues some of which could promote clotting. We’ve discussed the role of molecular mimicry in a previous article as well as the study by Vodjani and colleagues, which found that the components of the COVID virus reacted with 28 out of 55 human tissues, many of which were located in the brain. You can read more about the impacts of long COVID on brain tissue and the complex mechanisms at: https://www.suzannegazdamd.com/blog---long-covid/long-covid-and-the-brain-what-happens-when-the-storm-passes Chronic neuroinflammation and molecular mimicry plays an important role in the onset and progression of autoimmune disease and neurodegenerative diseases such as Alzheimer's disease (AD), Parkinson's disease (PD), Multiple Sclerosis (MS) and causes brain fog in long COVID. We have also learned that spike protein alone is toxic to the brain and body and can disrupt the BBB which may partly explain neurological injuries after the COVID vaccinations: Credit: CC BY creativecommons.org
No doubt, this persistent immune response to spike protein and the other downstream negative effects from spike protein are bad news for the brain.
0 Comments
Your comment will be posted after it is approved.
Leave a Reply. |
AuthorSuzanne Gazda M.D. Neurologist Archives
January 2024
Categories |

 RSS Feed
RSS Feed