|
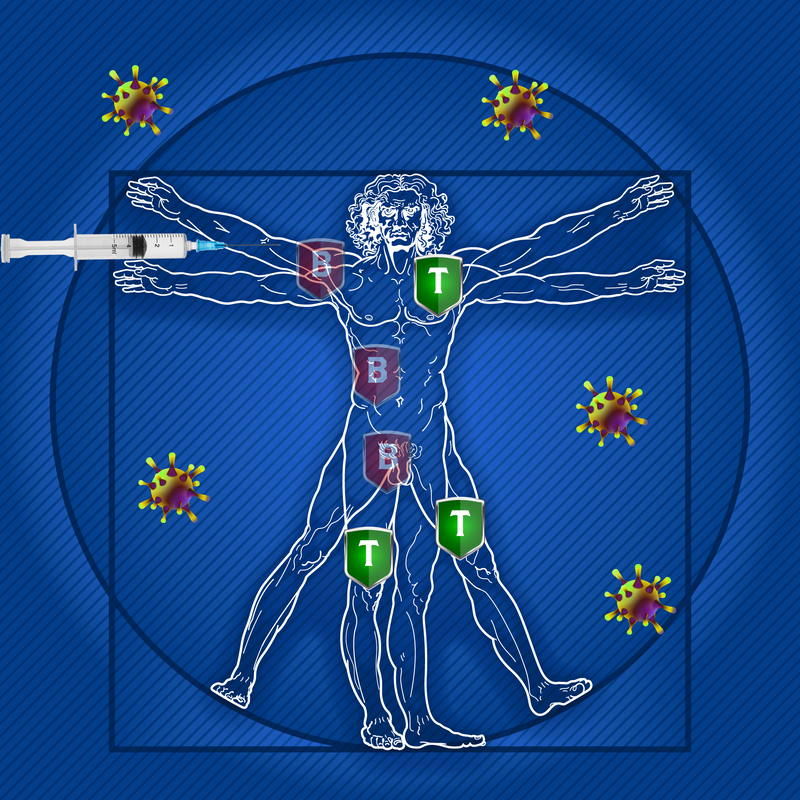
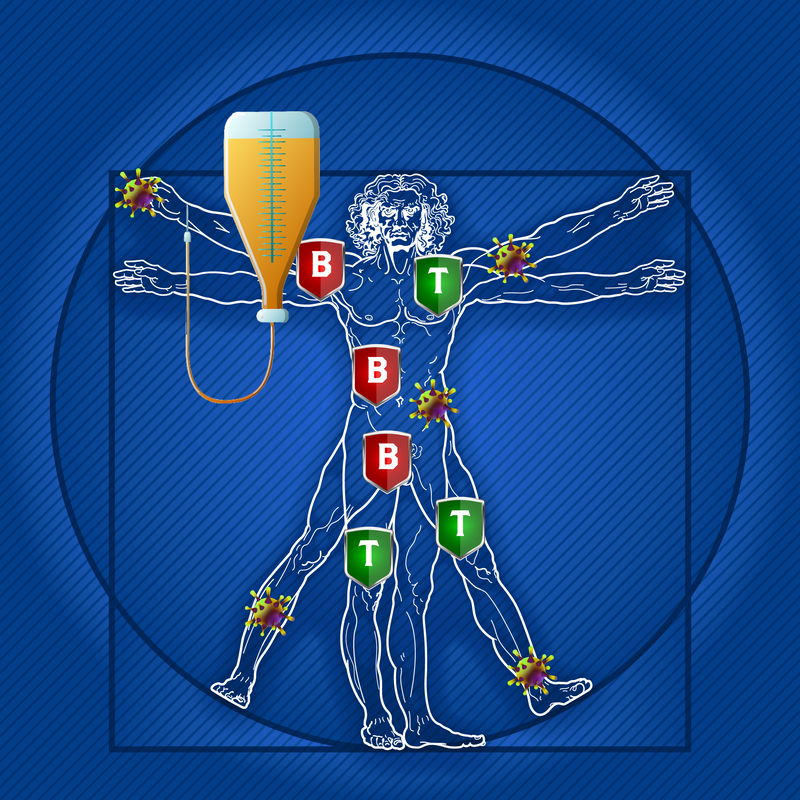
Of recent note are findings from a study designed to assess the impact of disease modifying therapies on the severity of COVID 19 in people with multiple sclerosis (MS). Of the 844 COVID-afflicted patients, those with primary progressive MS (PPMS) and/or receiving B cell depletion therapy (ocrelizumab) were at the highest risk of severe disease; patients who had received methylprednisolone within one month of infection were also identified as having worse outcomes.1 We know that some MS drugs can lower immunity and subsequently carry a risk of infection and attenuation of a vaccine response. Results from this study published in late 2020 that assessed immune response with administration of select non-live vaccines did show that despite an attenuated response, there would still be some protective action. While the study did look specifically at DMTs in MS, it’s helpful to understand their use in other autoimmune conditions as well. DMTs in neurology and rheumatology Normally, the immune system creates inflammation to protect the body from infections. Yet in some cases, a group of cells in the immune system, called B-cells, cause unnecessary inflammation which damages the body’s healthy tissue. Researchers have examined B cell functions in MS by focusing on their function as T cell activators and these cells influence immune responses when they differentiate into numerous antibody-producing plasma cells. However, they can, for example, also affect MS progression by stimulating T cells.2 Neurologists and rheumatologists use monoclonal antibodies (MABs) to target certain proteins (the CD20 antigen) found on the surface of B lymphocytes. MABs have become a mainstay of treatment for MS and some rheumatology-related diseases and are likely to continue to be developed and optimized for use, such as analyzing the safety of faster infusion times and administration by subcutaneous routes. And MABs are some of the most effective therapies for relapsing MS without the side effects associated with chemotherapeutic agents. There are a number of treatments in addition to infusion therapy such as subcutaneous injectable and oral medications. Depending on the type and current status of MS or a rheumatological disease, patients may be better candidates for some therapies than others – what helps one person may not be suitable or have value in treating someone else’s MS or rheumatoid arthritis, for example. What about DMTs and vaccines? There have been numerous studies to date regarding vaccine efficacy when patients are also receiving DMTs. These medications for MS and rheumatological diseases can impact or weaken your body’s ability to mount an effective immune response to a vaccine. Most trials looking at responses to vaccines (e.g. flu, pneumonia vaccines) in MS patients measured antibodies in the blood, which is driven by B cell immune response. You may be able to mount an effective T cell response to a virus, but this is much harder to measure. And we know we need both strong T and B cell responses for a vaccine to work if patients opt to receive available COVID vaccines. So the question then is whether these necessary therapies may impact the protective qualities of a COVID vaccine? There is some thinking that indicates timing of when to give a vaccine based on a scheduled course of treatment could help as far as improving efficacy. In the VELOCE trial, for example, MS patients receiving DMTs were studied for their response to various vaccines; antibody response rate to vaccines for influenza and pneumonia was clearly reduced compared to the placebo or interferon. IVIG as a safe and effective DMT for MS as well as rheumatology conditions. Studies continue to support the use of intravenous immunoglobulin (IVIG) as a historically safe and highly effective DMT for MS and rheumatology patients given its effects on the immune system that can have a beneficial influence on disease processes and symptoms. In MS, IVIG may be valuable in the treatment of acute relapses and in prevention of new relapses by promoting re-myelination and positively impacting rates of disability and disease progression.3 IVIG also may impose less negative effects on vaccine efficacy if patients choose to receive one. Studies in the last ten years also have shown IVIG to be valuable in rheumatology conditions too where B-cell depletion can decrease disease symptoms such as joint pain, swelling and stiffness. Rituximab, an anti-CD 20 drug used in both rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE), has proven to be safe and efficacious. In RA, complete B-cell depletion after rituximab treatment is associated with clinical response, and this outcome often leads to long-term maintenance of therapy.4 Some studies note that widespread adoption of monitoring before and after administration is needed to identify patients at risk of developing prolonged hypogammaglobulinanemia, which is a problem with the immune system that prevents it from making enough antibodies called immunoglobulins. In SLE, early treatment after diagnosis with rituximab “is safe, effective and enables a reduction in steroid use” and offers an option for patients whose response to or tolerance for other drug therapies is poor.5 What’s more, in recent months too we’ve seen that IVIG has excellent therapeutic value as a treatment for active COVID infections.6 What’s next?
There are naturally many questions for which we don’t necessarily have all the answers. Pre-COVID, neurologists’ and rheumatologists’ options for effective DMTs were more diverse, but now we must consider unrecognized immune deficiency, treatment impact on immunoglobulins, the impact of COVID severity from treatment effect AND also deal with concerns related to vaccine responses (both short and long term). And some choices may result in ineffective protection in the face of an ongoing pandemic with new mutations. The potential for severe virus outcomes for people with autoimmune diseases like MS, RA and SLE has to be weighed against the use of any treatment as well as the administration of vaccines for which all the data is not available, due simply to how rapidly this crisis has evolved. Patients must be fully informed about all the factors involved in any choice, whether to continue their current therapies, consider delaying their scheduled treatment and/or opting for or declining receipt of a vaccine. These are complex considerations for all general and specialty clinicians and patients alike so it’s critical to talk to each patient about their options as well as the value of integrative medicine’s diverse disciplines. Perhaps now more than ever, this expanded perspective and access to innovative protocols in neurological healthcare has never been more important. In health and hope, Dr. Suzanne Gazda References: 1 Sormani, M. P., De Rossi, N., Schiavetti, I., Carmisciano, L., Cordioli, C., Moiola, L., Radaelli, M., Immovilli, P., Capobianco, M., Trojano, M., Zaratin, P., Tedeschi, G., Comi, G., Battaglia, M. A., Patti, F., Salvetti, M., & Musc-19 study group (2021). Disease modifying therapies and Covid-19 severity in Multiple Sclerosis. Annals of neurology, 10.1002/ana.26028. Advance online publication. https://doi.org/10.1002/ana.26028 2 Arneth, B. Impact of B cells to the pathophysiology of multiple sclerosis. J Neuroinflammation 16, 128 (2019). https://doi.org/10.1186/s12974-019-1517-1 3 Sorensen PS. The role of intravenous immunoglobulin in the treatment of multiple sclerosis. J Neurol Sci. 2003 Feb 15;206(2):123-30. doi: 10.1016/s0022-510x(02)00343-x. PMID: 12559498. 4 Leticia Garcia-Montoya, Catalina Villota-Eraso, Md Yuzaiful Md Yusof, Edward M Vital, Paul Emery. Lessons for rituximab therapy in patients with rheumatoid arthritis. The Lancet Rheumatology, Volume 2, Issue 8, 2020, Pages e497-e509. ISSN 2665-9913, https://doi.org/10.1016/S2665-9913(20)30033-3. https://www.sciencedirect.com/science/article/pii/S2665991320300333 5 Gracia-Tello B, Ezeonyeji A, Isenberg D. The use of rituximab in newly diagnosed patients with systemic lupus erythematosus: long-term steroid saving capacity and clinical effectiveness Lupus Science & Medicine 2017;4:e000182. doi: 10.1136/lupus-2016-000182 6 Galeotti, C., Kaveri, S.V. and Bayry, J. (2020), Intravenous immunoglobulin immunotherapy for coronavirus disease‐19 (COVID‐19). Clin. Transl. Immunol., 9: e1198. https://doi.org/10.1002/cti2.1198
0 Comments
Your comment will be posted after it is approved.
Leave a Reply. |
AuthorDr. Suzanne Gazda, Integrative Neurology Archives
February 2024
Categories |
 RSS Feed
RSS Feed