|
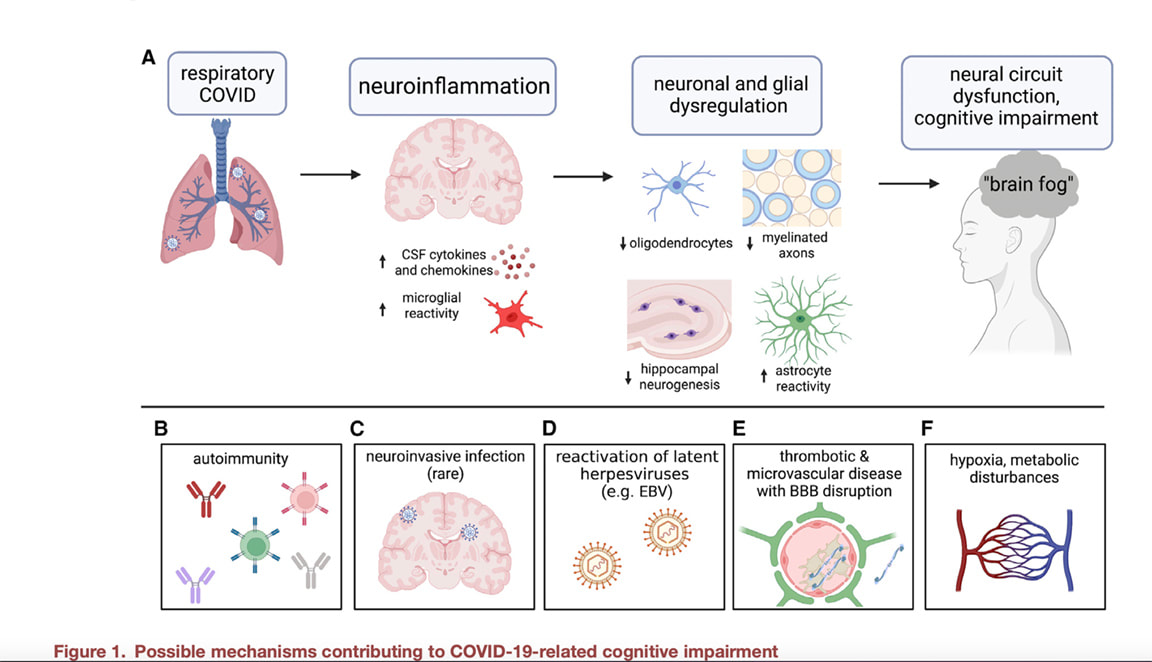
Multiple studies have now reported new onset or worsening multiple sclerosis (MS) in some patients after receiving one of the COVID vaccinations. This most recent paper discussing a patient who developed MS after a COVID vaccine also shares an in-depth review of other reported cases. Yet another recent paper presents a case of anti-myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD) reported after the vaccination in a young woman. MOGAD is a rare autoimmune demyelinating disorder that mainly occurs post-infection or post-vaccination. In May of last year, 23 cases of MOGAD after inoculation with several types of SARS-CoV-2 vaccines were reported; there are no reported cases of MOGAD related to the SARS COV 2 infection. Speaking from the front lines of a MS clinic in Texas, I remained very concerned about my MS patients and here is why: MS is caused by an autoimmune reaction against the CNS myelin and the myelin-producing oligodendrocytes; note too that MS also has strong vascular constituents. In a paper published by Dr. Michelle Monje and her team in the October issue of Cell, the results showed high levels of neuroinflammation driven by chemical messengers called cytokines with damage to cells in the brain that make myelin (oligodendrocytes) and white matter (the white matter is described as the lines of communication in the CNS). Myelin is a protective coating that helps nerve transmission be more effective and efficient. This study also found damage to the hippocampus or the primary memory center of the brain. Following mild respiratory COVID-19 in mice, pro-inflammatory CSF cytokines/chemokines were elevated for at least 7 weeks. These findings were induced by a mild respiratory SARS COV 2 in a mouse model. The Monje study found no evidence of virus in the mouse brain. These are the leading mechanistic theories of injury: 1. The immune response to SARS- CoV-2 in the respiratory system may cause neuroinflammation—increasing cytokines, chemokines, and immune cell trafficking in the brain and inducing reactive states of resident microglia and other immune cells in the brain and brain borders. Research has shown that spike protein alone can induce high levels of systemic and neuroinflammation and as well provoke a cascade of other downstream affects to the body and brain. A study, showing the downfall of immune health after the vaccine , “the BNT162b2 mRNA vaccine against SARS-CoV-2 reprograms both adaptive and innate immune responses,” while not yet peer reviewed, showed that the vaccines altered the production of inflammatory cytokines by innate immune cells following stimulation with both specific (SARS-CoV-2) and non-specific (viral, fungal and bacterial) stimuli. Following vaccination, innate immune cells had a reduced response to toll-like receptor 4 (TLR4), TLR7 and TLR8 – all ligands that play an important role in the immune response to viral infection and tumor surveillance. Toll-like receptors are also involved in the pathogenesis of MS and play a major role in the initiation of disease. 2. Viral fragments such as spike protein cross the BBB and elicit SARS-CoV-2 spike S1 induced neuroinflammation, microglial and behavioral sickness responses. Neuroinflammation is triggered by microglial cell activation (the brain’s innate immune cells). Microglial reactivity leads to secretion of cytokines and enhanced phagocytosis that is intended to limit the spread of pathogens, but when not properly regulated, can profoundly disrupt neural circuit regulation, function, and plasticity in ways that can contribute to central nervous system (CNS) disease. Reactive microglia can inhibit neurogenesis (the process by which new neurons are formed in the brain) directly through secretion of cytokines and can negatively influence myelinating oligodendrocytes in multiple ways, including indirectly by inciting neurotoxic astrocyte reactivity that can kill oligodendrocytes through toxic lipid secretion and by reducing neuronal brain-derived neurotrophic factor (BDNF) expression, which is required for the neuronal survival and myelin plasticity that contributes to attention, memory, and learning. 3. Spike protein causes Increased thrombosis and microclotting leading to more break down of the BBB and more neuroinflammation. 4. Molecular mimicry to spike protein and other viral fragments SARS-CoV-2 have been widely demonstrated to elicit strong immune responses. The spike protein’s sequence has a high degree of overlap with many different human tissues. When this overlap is present, autoimmune responses to the matching protein often initiate (termed autoimmune mimicry). In the paper by Vojdany et al, myelin shared molecular mimicry to spike protein. Additionally, an Israeli study found that 24.2% of those receiving a booster developed an exacerbation of a pre-existing autoimmune condition. 5. Lipid nanoparticles are used to encase and protect the mRNA in the COVID vaccines They have been found to be highly inflammatory and they themselves can alter the immune system. Lipid nanoparticles easily cross the into the brain What Other Factors Might be Causing New Onset or Worsening Demyelinating Disease MS Could Be Triggered by COVID-19 Via 'Molecular Mimicry' Nucleocapsid of seasonal coronaviruses show homology with MS-associated proteins, but only SARS-CoV-2 overlaps with PLP. Credit: https://coronavirusexplained.ukri.org/en/article/cad0010/ Based on this study published in Nature, findings noted a higher degree of molecular mimicry of the SARS-CoV-2 nucleocapsid (N) protein and MS proteins like myelin. The authors of this paper found a higher degree of peptide similarity between SARS-CoV-2 N and MS-associated proteins, than for 3 out of 4 seasonal coronaviruses that also had significant peptide similarities. Once again, we ponder why SARS COV 2 has these unique properties? Seasonal coronaviruses also have been implicated in the development of MS but what we are seeing develop over the last 2 plus years is truly concerning with multiple case reports of new or worsening MS. There are very few case reports of MS reported during the first SARS outbreak. In summary Dr. Monje’s work sheds light on the complex neurobiological mechanisms involved in long COVID and clearly showed that the oligodendrocytes, myelin, white matter and the hippocampus are in line of fire following an infection, and potentially after the vaccines if spike and other viral proteins linger. What does this mean for MS patients who already have an autoimmune disease affecting oligodendrocytes, myelin, and white matter? The cumulative toxic effect on neurological tissue from chronic exposure to toxic viral fragments elicited by infection and/or repeated periodic mRNA injections is not known. The phrase, “canary in the coal mine” comes to mind, often used to connote early warning signs of danger that may not be immediately visible.
In addition to the many articles we have available in our blog archives regarding long COVID, spike protein, and vaccines, we have included a list of journal articles below to provide you with references and additional subject matter reading. As always, we invite you to reach out to our offices if you have questions or would like to schedule an appointment. In hope and healing, Dr. Suzanne Gazda References and additional reading: Rinaldi V, Bellucci G, Buscarinu MC, et al. CNS inflammatory demyelinating events after COVID-19 vaccines: A case series and systematic review. Front Neurol. 2022;13:1018785. Published 2022 Dec 1. doi:10.3389/fneur.2022.1018785. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7246018/?utm_source=substack&utm_medium=email Al-Allaf AW, Neethu M, Al-Allaf Y. A Case Series and Literature Review of the Association of COVID-19 Vaccination With Autoimmune Diseases: Causality or Chance?. Cureus. 2022;14(9):e28677. Published 2022 Sep 1. doi:10.7759/cureus.28677 Theoharides TC. Could SARS-CoV-2 Spike Protein Be Responsible for Long-COVID Syndrome? Mol Neurobiol. 2022 Mar;59(3):1850-1861. doi: 10.1007/s12035-021-02696-0. Epub 2022 Jan 13. PMID: 35028901; PMCID: PMC8757925 Shankar R, Joshi M, Pathak K. Lipid Nanoparticles: A Novel Approach for Brain Targeting. Pharm Nanotechnol. 2018;6(2):81-93. doi:10.2174/2211738506666180611100416 Miranda-Hernandez S, Baxter AG. Role of toll-like receptors in multiple sclerosis. Am J Clin Exp Immunol. 2013;2(1):75-93. Published 2013 Feb 27. Qin Z, Bouteau A, Herbst C, Igyártó BZ. Pre-exposure to mRNA-LNP inhibits adaptive immune responses and alters innate immune fitness in an inheritable fashion. PLoS Pathog. 2022;18(9):e1010830. Published 2022 Sep 2. doi:10.1371/journal.ppat.1010830 Seneff S, Nigh G, Kyriakopoulos AM, McCullough PA. Innate immune suppression by SARS-CoV-2 mRNA vaccinations: The role of G-quadruplexes, exosomes, and MicroRNAs. Food Chem Toxicol. 2022;164:113008. doi:10.1016/j.fct.2022.113008 Fernández-Castañeda A, Lu P, Geraghty AC, et al. Mild respiratory COVID can cause multi-lineage neural cell and myelin dysregulation. Cell. 2022;185(14):2452-2468.e16. doi:10.1016/j.cell.2022.06.008 Fernández-Castañeda A, Lu P, Geraghty AC, et al. Mild respiratory COVID can cause multi-lineage neural cell and myelin dysregulation. Cell 2022 Protein expression profile of ACE2 in the normal and COVID-19-affected human brain. Lindsong C et al J Proteome Res. 2022 SARS-CoV-2 spike protein induces cognitive deficit and anxiety-like behavior in mouse via non-cell autonomous hippocampal neuronal death https://pubmed.ncbi.nlm.nih.gov/35361832/ Clough E, Inigo J, Chandra D, et al. Mitochondrial Dynamics in SARS-COV2 Spike Protein Treated Human Microglia: Implications for Neuro-COVID [published correction appears in J Neuroimmune Pharmacol. 2021 Dec 11;:]. J Neuroimmune Pharmacol. 2021;16(4):770-784. doi:10.1007/s11481-021-10015-6 Yang AC, Kern F, Losada PM, et al. Dysregulation of brain and choroid plexus cell types in severe COVID-19 [published correction appears in Nature. 2021 Oct;598(7882):E4]. Nature. 2021;595(7868):565-571. doi:10.1038/s41586-021-03710-0 Lee, M. et al. Microvascular Injury in the Brains of Patients with Covid-19. NEJM. Feb 2021 https://www.nejm.org/doi/full/10.1056/NEJMc2033369 Nikano, H. et al. Relapsing Anti-MOG Antibody-associated Disease Following COVID-19 Vaccination: A Rare Case Report and Review of the Literature. (2022) https://www.jstage.jst.go.jp/article/internalmedicine/advpub/0/advpub_0504-22/_pdf/-char/en Seneff, S. et al. MAPK Activation, P53 and Autophagy Inhibition Characterize the SARS-CoV-2 Spike Protein Induced Neurotoxicity. https://www.authorea.com/users/455597/articles/595736-mapk-activation-p53-and-autophagy-inhibition-characterize-the-sars-cov-2-spike-protein-induced-neurotoxicity Abu-Abaa M, Dawood G, Arshad H, et al. (November 05, 2022) A Possible Case of Autoimmune Encephalitis After mRNA COVID-19 Booster Vaccine: A Case Report. Cureus 14(11): e31118. doi:10.7759/cureus.31118 Al-Allaf A, Neethu M, Al-Allaf Y (September 01, 2022) A Case Series and Literature Review of the Association of COVID-19 Vaccination With Autoimmune Diseases: Causality or Chance?. Cureus 14(9): e28677. doi:10.7759/cureus.28677. https://www.cureus.com/articles/108871-a-case-series-and-literature-review-of-the-association-of-covid-19-vaccination-with-autoimmune-diseases-causality-or-chance Song, E. et al. Neuroinvasion of SARS-CoV-2 in human and mouse brain. J Exp Med 1 March 2021; 218 (3): e20202135. doi: https://doi.org/10.1084/jem.20202135 Olajide OA, Iwuanyanwu VU, Adegbola OD, Al-Hindawi AA. SARS-CoV-2 Spike Glycoprotein S1 Induces Neuroinflammation in BV-2 Microglia. Mol Neurobiol. 2022;59(1):445-458. doi:10.1007/s12035-021-02593-6 Nyström S, Hammarström P. Amyloidogenesis of SARS-CoV-2 Spike Protein. J Am Chem Soc. 2022;144(20):8945-8950. doi:10.1021/jacs.2c03925 Patel J, Balabanov R. Molecular mechanisms of oligodendrocyte injury in multiple sclerosis and experimental autoimmune encephalomyelitis. Int J Mol Sci. 2012;13(8):10647-10659. doi:10.3390/ijms130810647 Monje M, Iwasaki A. The neurobiology of long COVID. Neuron. 2022;110(21):3484-3496. doi:10.1016/j.neuron.2022.10.006 Havla J, Schultz Y, Zimmermann H, Hohlfeld R, Danek A, Kümpfel T. First manifestation of multiple sclerosis after immunization with the Pfizer-BioNTech COVID-19 vaccine. J Neurol. 2022;269(1):55-58. doi:10.1007/s00415-021-10648-w Etemadifar M, Sigari AA, Sedaghat N, Salari M, Nouri H. Acute relapse and poor immunization following COVID-19 vaccination in a rituximab-treated multiple sclerosis patient. Hum Vaccin Immunother. 2021;17(10):3481-3483. doi:10.1080/21645515.2021.1928463 Nabizadeh F, Ramezannezhad E, Kazemzadeh K, Khalili E, Ghaffary EM, Mirmosayyeb O. Multiple sclerosis relapse after COVID-19 vaccination: A case report-based systematic review. J Clin Neurosci. 2022;104:118-125. doi:10.1016/j.jocn.2022.08.012 Sokratis, A. et al. Altered cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. MedRxiv. https://www.medrxiv.org/content/10.1101/2021.06.23.21259389v1 Youn T, Yang H. Cytotoxic Lesion of the Corpus Callosum (CLOCCs) after SARS-CoV-2 mRNA Vaccination. J Korean Med Sci. 2021;36(31):e228. Published 2021 Aug 9. doi:10.3346/jkms.2021.36.e228 Seyed Ahadi M, Ghadiri F, Ahraian MA, Naser Moghadasi A. Acute attack in a patient with multiple sclerosis 2 days after COVID vaccination: a case report [published online ahead of print, 2021 Aug 11]. Acta Neurol Belg. 2021;1-2. doi:10.1007/s13760-021-01775-2 Khayat-Khoei M, Bhattacharyya S, Katz J, et al. COVID-19 mRNA vaccination leading to CNS inflammation: a case series. J Neurol. 2022;269(3):1093-1106. doi:10.1007/s00415-021-10780-7 Toljan K, Amin M, Kunchok A, Ontaneda D. New diagnosis of multiple sclerosis in the setting of mRNA COVID-19 vaccine exposure. J Neuroimmunol. 2022;362:577785. doi:10.1016/j.jneuroim.2021.577785 Maramattom BV. Myelin Oligodendrocyte Glycoprotein-Associated Disorders Post-ChAdOx1 Vaccination. Cureus. 2022;14(3):e23197. Published 2022 Mar 15. doi:10.7759/cureus.23197 Lake CM, Breen JJ. Sequence similarity between SARS-CoV-2 nucleocapsid and multiple sclerosis-associated proteins provides insight into viral neuropathogenesis following infection. Sci Rep. 2023;13(1):389. Published 2023 Jan 8. doi:10.1038/s41598-022-27348-8 https://pubmed.ncbi.nlm.nih.gov/36617594/ Choi Y et al: Nanoparticles for gene delivery: therapeutic and toxic effects Molecular & Cellular Toxicology volume 10, pages 1–8 (2014)
0 Comments
Your comment will be posted after it is approved.
Leave a Reply. |
AuthorSuzanne Gazda M.D. Neurologist Archives
January 2024
Categories |


 RSS Feed
RSS Feed