|
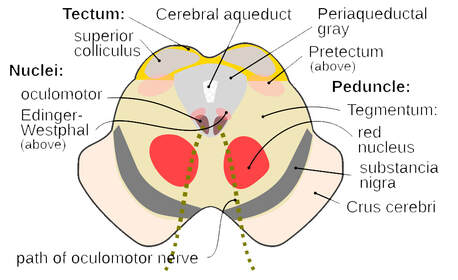
Intractable headaches are defined as persistent headache that is difficult to treat or fails to respond to standard and/or aggressive treatment modalities. It’s important to note that headache pain is identified as the third most common symptom in long COVID cases – and for those patients who previously experienced migraines, the headache often becomes even more difficult to treat. So, what mechanisms can contribute to this? We’ve previously discussed at length the potential causes – read more about the science here: https://www.suzannegazdamd.com/blog---long-covid/long-haulers-syndrome-understanding-the-mechanisms-behind-resulting-headaches But we know that spike protein shares molecular mimicry with the CGRP (calcitonin gene-related peptide) receptors, which can then incite high levels of neuroinflammation, triggering the release of more glutamate and mast cells. And this in turn fosters more endothelial dysfunction. A new study sheds additional light on why so many patients suffer with headaches after recovering from COVID. Findings noted structural changes in the periaqueductal gray (PAG), a key structure in the propagation and modulation of pain and sympathetic responses, as well as the learning and action of defensive and aversive behavior. Damage in the PAG has been associated with causing headaches. These pathways are involved in the descending control of trigeminovascular nociceptive traffic. A trigeminovascular system is often implicated as integral to the pain, inflammation, and secondary vascular effects of migraine, linked through the NMDA/glutamate system. Damage here will cause dysregulation of pain pathways that could include the release of an onslaught of glutamate and other neurotransmitters. In the study they found the combined glutamine and glutamate/total creatine ratio (Glx/tCr) was increased in the PAG following COVID-19 infection. Image credit: Wikipedia, https://en.wikipedia.org/wiki/Periaqueductal_gray Several lines of evidence indicate that both glutamate and CGRP (as my prior blog noted) are important neurotransmitters in migraine and many other neurological and psychiatric symptoms and disease states. The most abundant neurotransmitter in our brains, excessive amounts of glutamate are thought to be linked to the following conditions:
The role of glutamate in some disorders. As the primary excitatory neurotransmitter in the brain, glutamate is present in about 50% of synapses, which are the small gaps at the end of a neuron that allow a signal to pass from one neuron to the next. Glutamatergic neurotransmission has a fundamental role for neuronal plasticity, learning, and memory. However, in pathological conditions, glutamate also can act as a neuronal excitotoxin, leading to rapid or delayed neurotoxicity. As noted above, glutamate system dysfunction that leads to overproduction and overstimulation of brain cells has been linked to many psychological and neurodegenerative disorders and sometimes to the point of death. The relationship between glutamate and GABA. You really can’t discuss glutamate without also mentioning GABA (gamma-aminobutyric acid), another abundant neurotransmitter that lessens a nerve cell's ability to receive, create or send chemical messages to other nerve cells. Studies have shown that glutamate is the precursor of GABA. Glutamate and GABA are integrally related in both form and function. They have a complex, homeostatic relationship that brings balance to the level of brain activity:
You can read more about the complex relationship of these two neurotransmitters here: https://www.suzannegazdamd.com/blog---ms-in-the-news/the-relationship-between-glutamate-and-gaba Image courtesy of University of Utah There are two main causes of excess glutamate and its resulting excitotoxicity:
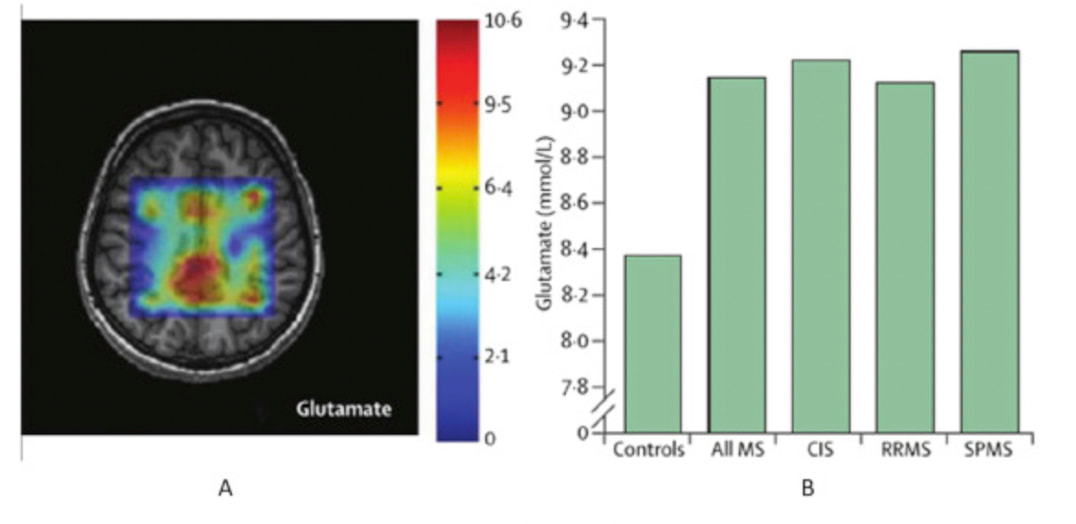
Receptor oversensitivity sometimes occurs in patients with neurodegenerative disorders even when glutamate levels are not particularly high. Symptoms indicative of an elevated level of glutamate include anxiety, depression, restlessness, inability to concentrate, headaches, insomnia, fatigue, and increased sensitivity to pain. Chronic excitotoxicity due to a persistent excess of glutamate is a key element in neurodegenerative conditions such as Alzheimer’s, Parkinson’s, multiple sclerosis, and other disorders. Using sophisticated imaging processes (depicted in the following visuals) to better understand disease progression in MS, scientists identified glutamate concentrations had increased in active, enhancing lesions, and remained normal by contrast in chronic lesions infiltrated by inflammation. According to these researchers, other experimental studies also suggest that increased inflammation is likely related to the presence of glutamate in active lesions because these types of lesions show high glutaminase expression. Therefore, conducting imaging in diagnosed MS patients could prove extremely valuable for detecting changes in glutamate concentration that imply inflammation has already begun to impact the central nervous system. And earlier detection could help direct more effective treatment strategies! Glutamate concentrations in multiple sclerosis. In Glutamate concentrations (mmol/L) in the white matter shown here, with differences between patients and healthy controls. (A) Chemical-shift imaging using echo time (TE)-average point resolved spectroscopy (PRESS) at 3T, which is an editing technique used to highlight glutamate signal; warmer colors show higher glutamate concentrations, whereas cooler colors show lower metabolite concentrations. (B) Differences in glutamate concentrations between all patients with multiple sclerosis, each multiple sclerosis group, and healthy controls. MS = multiple sclerosis. SPMS = secondary-progressive multiple sclerosis. RRMS = relapsing-remitting multiple sclerosis. CIS = clinically isolated syndrome Source: Chen, Y. et al. Magnetic resonance imaging of glutamate in neuroinflammation Radiology of Infectious Disease. 2016. https://www.sciencedirect.com/science/article/pii/S235262111530015 How to restore the balance of glutamate levels.
There are several ways that we can help reduce or block glutamate when it is out of balance. For example, I previously detailed the effects of glutamate in MS progression and the potential for use of memantine in disease therapies. In addition to memantine, which is prescribed for its properties that may reduce abnormal brain activity, there are other glutamate-blocking medications such as:
The N-methyl-D-aspartate (NMDA) receptor is a receptor of glutamate, the primary excitatory neurotransmitter in the human brain. It plays an integral role in synaptic plasticity, which is a neuronal mechanism believed to be the basis of memory formation. Glutamate-blocking supplements. In addition to the use of prescription medications, there are supplements that may also positively impact the levels of glutamate. Note that as with any other type of supplement, always choose a high-quality product from a reputable source to ensure it contains the active ingredient.
Magnesium concentration influences serotonin receptors, nitric oxide synthesis and release, NMDA receptors, and a variety of other migraine related receptors and neurotransmitters. The available evidence suggests that up to 50% of patients during an acute migraine attack have lowered levels of ionized magnesium. Dosage - 400-600 mg daily
What about lifestyle modifications? When it comes to headache occurrence, there are steps you can take that may help improve your condition and symptoms. Consider these factors in your overall therapeutic approach:
Following a dietary approach that limits glutamate is vital to potentially reducing the incidence of headaches. Dr. Josh Axe, DC, DNM, CNS, is a certified clinical nutritionist who wrote an excellent piece that covers what glutamate-containing foods to avoid: https://draxe.com/nutrition/glutamate/ You may also want to look at the program by Sue Kira, a well-known naturopath and nutritionist: https://www.truevitality.com.au/low-glutamate-diet-by-sue-kira/
Intractable headaches can be just one symptom of a disorder, whether migraine or long COVID as well as another neurological or autoimmune condition. When devising a comprehensive treatment protocol, it’s important to identify the root cause of which excessive glutamate may be a significant contributing component. That’s why our clinic works with each patient to fully understand your individual needs and to make recommendations that are most appropriate for your health. We invite you to please check out our extensive blog library with multiple resources and more information and don’t hesitate to reach out to our offices if we can help! In hope and healing, Dr. Suzanne Gazda References: Hoffman, J. et al. Glutamate and Its Receptors as Therapeutic Targets for Migraine. Neurotherapeutics. 2008 Petroff OA. GABA and glutamate in the human brain. Neuroscientist. 2002;8(6):562-573.doi:10.1177/1073858402238515 Learn Genetics. What is a brain pathway? University of Utah.https://learn.genetics.utah.edu/content/addiction/brainpathways/ (retrieved 1/13/2024) Holten AT, Gundersen V. Glutamine as a precursor for transmitter glutamate, aspartate and GABA in the cerebellum: a role for phosphate-activated glutaminase. J Neurochem. 2008;104(4):1032-1042. doi:10.1111/j.1471-4159.2007.05065.x Huntington’s Outreach Project for Education. HOPES Stanford University. 2020. https://hopes.stanford.edu/about-glutamate-toxicity/ Goadsby PJ, Holland PR, Martins-Oliveira M, Hoffmann J, Schankin C, Akerman S. Pathophysiology of Migraine: A Disorder of Sensory Processing. Physiol Rev. 2017;97(2):553-622. doi:10.1152/physrev.00034.2015 Xiao Y, Richter JA, Hurley JH. Release of Glutamate and CGRP from Trigeminal Ganglion Neurons: Role of Calcium Channels and 5-HT1 Receptor Signaling. Molecular Pain. 2008;4. doi:10.1186/1744-8069-4-12 Chen, Y. et al. Magnetic resonance imaging of glutamate in neuroinflammation Radiology of Infectious Disease. 2016. https://www.sciencedirect.com/science/article/pii/S2352621115300152 Nakano T, Hasegawa T, Suzuki D, Motomura E, Okada M. Amantadine Combines Astroglial System Xc- Activation with Glutamate/NMDA Receptor Inhibition. Biomolecules. 2019;9(5):191. Published 2019 May 17. doi:10.3390/biom9050191 Jewett, B., Thapa, B. Physiology – NMDA Receptor. National Library of Medicine, Stat Pearls. 2022. https://www.ncbi.nlm.nih.gov/books/NBK519495/ Blanpied TA, Clarke RJ, Johnson JW. Amantadine inhibits NMDA receptors by accelerating channel closure during channel block. J Neurosci. 2005;25(13):3312-3322. doi:10.1523/JNEUROSCI.4262-04.2005 Leng Y, Fessler EB, Chuang DM. Neuroprotective effects of the mood stabilizer lamotrigine against glutamate excitotoxicity: roles of chromatin remodelling and Bcl-2 induction. Int J Neuropsychopharmacol. 2013;16(3):607-620. doi:10.1017/S1461145712000429 Gensel JC, Tovar CA, Bresnahan JC, Beattie MS (2012) Topiramate Treatment Is Neuroprotective and Reduces Oligodendrocyte Loss after Cervical Spinal Cord Injury. PLoS ONE. 7(3): e33519. https://doi.org/10.1371/journal.pone.0033519 Ueda Y, Doi T, Tokumaru J, Willmore LJ. Effect of zonisamide on molecular regulation of glutamate and GABA transporter proteins during epileptogenesis in rats with hippocampal seizures. Brain Res Mol Brain Res. 2003;116(1-2):1-6. doi:10.1016/s0169-328x(03)00183-9 Marashly ET, Bohlega SA. Riboflavin Has Neuroprotective Potential: Focus on Parkinson's Disease and Migraine. Front Neurol. 2017;8:333. Published 2017 Jul 20. doi:10.3389/fneur.2017.00333 Field DT, Cracknell RO, Eastwood JR, et al. High-dose Vitamin B6 supplementation reduces anxiety and strengthens visual surround suppression. Hum Psychopharmacol. 2022;37(6):e2852. doi:10.1002/hup.2852 Ye HB, Shi HB, Yin SK. Mechanisms underlying taurine protection against glutamate-induced neurotoxicity. Can J Neurol Sci. 2013;40(5):628-634. doi:10.1017/s0317167100014840 Lee D, Shim MS, Kim KY, et al. Coenzyme Q10 inhibits glutamate excitotoxicity and oxidative stress-mediated mitochondrial alteration in a mouse model of glaucoma. Invest Ophthalmol Vis Sci. 2014;55(2):993-1005. Published 2014 Feb 18. doi:10.1167/iovs.13-12564 Zimmerman MA, Hall M, Qi Q, Mehta SL, Chen G, Li PA. Ubisol Coenzyme Q10 promotes mitochondrial biogenesis in HT22 cells challenged by glutamate. Exp Ther Med. 2021;22(5):1295. doi:10.3892/etm.2021.10730 Lin TY, Lu CW, Chang CC, Huang SK, Wang SJ. Luteolin inhibits the release of glutamate in rat cerebrocortical nerve terminals. J Agric Food Chem. 2011;59(15):8458-8466. doi:10.1021/jf201637u Lu CW, Lin TY, Wang SJ. Quercetin inhibits depolarization-evoked glutamate release in nerve terminals from rat cerebral cortex. Neurotoxicology. 2013;39:1-9. doi:10.1016/j.neuro.2013.07.009 Cheng Z, Kang C, Che S, et al. Berberine: A Promising Treatment for Neurodegenerative Diseases. Front Pharmacol. 2022;13:845591. Published 2022 May 20. doi:10.3389/fphar.2022.845591 Arcusa R, Villaño D, Marhuenda J, Cano M, Cerdà B, Zafrilla P. Potential Role of Ginger (Zingiber officinale Roscoe) in the Prevention of Neurodegenerative Diseases. Front Nutr. 2022;9:809621. Published 2022 Mar 18. doi:10.3389/fnut.2022.809621 Chen K, An Y, Tie L, Pan Y, Li X. Curcumin Protects Neurons from Glutamate-Induced Excitotoxicity by Membrane Anchored AKAP79-PKA Interaction Network. Evid Based Complement Alternat Med. 2015;2015:706207. doi:10.1155/2015/706207 Moldzio R, Radad K, Krewenka C, Kranner B, Duvigneau JC, Rausch WD. Protective effects of resveratrol on glutamate-induced damages in murine brain cultures. J Neural Transm (Vienna). 2013;120(9):1271-1280. doi:10.1007/s00702-013-1000-6 Zhang LN, Hao L, Wang HY, et al. Neuroprotective effect of resveratrol against glutamate-induced excitotoxicity. Adv Clin Exp Med. 2015;24(1):161-165. doi:10.17219/acem/38144 Savaskan NE, Bräuer AU, Kühbacher M, et al. Selenium deficiency increases susceptibility to glutamate-induced excitotoxicity. FASEB J. 2003;17(1):112-114. doi:10.1096/fj.02-0067fje Ikeda M, Azuma S, Inoué S. Vitamin B12 enhances GABA content but reduces glutamate content in the rat suprachiasmatic nucleus. Am J Physiol. 1997;273(1 Pt 2):R359-R363. doi:10.1152/ajpregu.1997.273.1.R359 Antony B, Merina B, Iyer VS, Judy N, Lennertz K, Joyal S. A Pilot Cross-Over Study to Evaluate Human Oral Bioavailability of BCM-95CG (Biocurcumax), A Novel Bioenhanced Preparation of Curcumin. Indian J Pharm Sci. 2008;70(4):445-449. doi:10.4103/0250-474X.44591 Nathan PJ, Lu K, Gray M, Oliver C. The neuropharmacology of L-theanine(N-ethyl-L-glutamine): a possible neuroprotective and cognitive enhancing agent. J Herb Pharmacother. 2006;6(2):21-30. Mallick HN. Understanding safety of glutamate in food and brain. Indian J Physiol Pharmacol. 2007;51(3):216-234. Cooper, J.A., Nuutinen, M.R., Lawlor, V.M. et al. Reduced adaptation of glutamatergic stress response is associated with pessimistic expectations in depression. Nat Commun 12, 3166 (2021). https://doi.org/10.1038/s41467-021-23284-9 Canadian Science Publishing (NRC Research Press). (2016, May 17). Using exercise to reduce glutamate build-up in the brain. ScienceDaily. Retrieved January 15, 2024 from www.sciencedaily.com/releases/2016/05/160517083040.htm Kleinkauf-Rocha J, Bobermin LD, Machado Pde M, Gonçalves CA, Gottfried C, Quincozes-Santos A. Lipoic acid increases glutamate uptake, glutamine synthetase activity and glutathione content in C6 astrocyte cell line. Int J Dev Neurosci. 2013;31(3):165-170. doi:10.1016/j.ijdevneu.2012.12.006
0 Comments
Your comment will be posted after it is approved.
Leave a Reply. |
AuthorSuzanne Gazda M.D. Neurologist Archives
January 2024
Categories |


 RSS Feed
RSS Feed