|
With long COVID linked to an increased risk of Alzheimer’s, gaining better understanding of the mechanisms is critical for better patient care.
For more than 40 years, researchers have widely believed that Alzheimer’s disease (AD) symptoms are largely due to a buildup of insoluble plaques of beta-amyloid in the brain. This is known as the amyloid cascade hypothesis. According to this hypothesis, soluble beta-amyloid protein is deposited and forms insoluble amyloid plaques, which damage neurons and synapses.
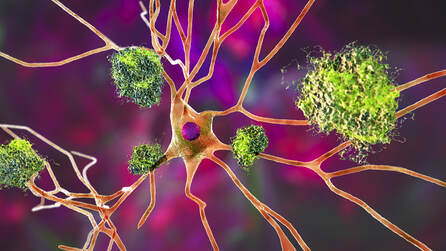
The amyloid hypothesis holds that Alzheimer’s results from a build-up of sticky, soluble proteins — amyloid-β peptides — in the spaces between brain cells. These peptides are cleaved from another protein embedded in the membranes of neurons; once floating free, they clump together into larger structures which, if not cleared efficiently enough by special enzymes, aggregate into plaques. The plaques then trigger a deadly cascade: they provoke neuroinflammation and spawn bundles of stringy proteins called tau tangles. Faced with this litany of insults, neurons die.
This impairs the normal transmission of nerve impulses, leading to typical dementia symptoms, such as memory loss, language problems, and unpredictable behavior. There is abundant evidence for the reactivity of microglia (microgliosis) in the pathogenesis of AD (microglia are the brains resident immune cells). The severity of cognitive impairment in AD correlates well with reactive gliosis and synaptic loss. In mice, prevention of microgliosis by minocycline rescues synaptic plasticity and cognitive behavior with little effect on Aβ levels.
Neuroinflammation is instigated by the misfiring of these immune cells in the central nervous system (CNS) involving microglia (and astrocytes). Neuroinflammation is a consequence of CNS injury, infection, toxicity, or autoimmunity – and chronic neuroinflammation is the downfall of the brain and potential instigator of many neurological diseases. What causes early-onset Alzheimer’s disease? Experts don't know what triggers the start of Alzheimer disease. We have been taught that this pathophysiological process may begin decades before clinical signs appear. A variety of toxic environmental risk factors including electromagnetic fields, solvents, pesticides, toxic metals, and air pollutants have been linked. Additional impacts include: heavy metals; pesticides; persistent organic pollutants; antibiotics; food additives; head trauma; post-traumatic stress; nanomaterials; alteration of the gut microbiome; poor nutrition; poor metabolic health; vascular disease; alcohol and smoking; lack of exercise; poor sleep; family history, age, gender, and more. For details, see: https://www.ttuhsc.edu/medicine/internal/research/documents/Mar_2020_LifeStyleAlzheimersDisease.pdf https://www.alz.org/alzheimers-dementia/what-is-alzheimers/causes-and-risk-factors Infectious elements might drive the risk as well, as shown in this study that reported infections requiring in-hospital treatment are associated with a long-term increased risk of dementia, including vascular dementia and Alzheimer's disease. Studies also suggest microbial organisms are involved such as SARS CoV 2, HSV1, and varicella virus. Two Pathological Proteins. The pathological hallmarks of Alzheimer’s disease consist of two proteins that damage and kill nerve cells. Fragments of one protein, beta-amyloid, build up and are called plaques. Twisted fibers of another protein, tau, are called tangles. Almost everyone develops plaques and tangles as they age, but patients with Alzheimer’s disease develop many, many more. At first, these plaques and tangles damage the memory areas of the brain and over time, are shown to affect additional areas of the brain as well.
In a new study published in the Journal of Alzheimer’s Disease, researchers report that people 65 and older who contracted COVID-19 were more prone to developing Alzheimer’s disease in the year following their COVID diagnosis. And the highest risk was observed in women at least 85 years old.
I have recently written about the association of increased risk of dementia related to a COVID infection. Given the mechanisms explored in this blog, I also propose, that this risk will also been seen in some patients with post-vaccine injuries. What are the symptoms of early-onset Alzheimer’s disease? For most people with early-onset Alzheimer’s disease, the symptoms closely mirror those of other forms of the condition. The early symptoms may include:
Later symptoms in the disease progression also can include:
How do we diagnose Alzheimer’s disease?
Obtain a clinical history
Ask the person experiencing symptoms, as well as a family member or friend, questions about overall health, use of prescription and over-the-counter medicines, diet, past or current medical problems, level of the patient’s ability to carry out daily activities, and observed changes in behavior and personality. A complete clinical history may include:
Identifying Early-Onset Alzheimer's Disease
The three single-gene mutations associated with early-onset Alzheimer’s disease are:
Mutations in these genes result in the production of abnormal proteins that are associated with the disease. Each of these mutations plays a role in the breakdown of APP, a protein whose precise function is not yet fully understood. This breakdown is part of a process that generates harmful forms of amyloid plaques, a hallmark of Alzheimer’s disease. Brain Imaging with MRI
Positron emission tomography (PET)
PET uses small amounts of a radioactive substance, called a tracer, to measure specific activity — such as energy use — or a specific molecule in different brain regions. PET scans take pictures of the brain, revealing regions of normal and abnormal chemical activity. There are several types of PET scans that can help doctors diagnose dementia.
In 2020, the FDA approved flortaucipir, the first diagnostic agent for measuring tau tangles on a PET scan of the brain. This approval is a major technological advance in biomarker tests for Alzheimer’s.
Cerebrospinal fluid biomarkers (CSF) CSF is a clear fluid that surrounds the brain and spinal cord, providing protection and insulation. CSF also supplies numerous nutrients and chemicals that help keep brain cells healthy. Proteins and other substances made by brain cells can be detected in CSF. Measuring changes in the levels of these substances can help diagnose neurological problems. It was shown that better results were obtained by identifying four key biomarkers: β-amyloid peptide 42 (Aβ42), Aβ40, total tau, and phosphorylated tau levels:
Blood Biomarkers Plasma biomarkers for Alzheimer's disease (AD) have broad potential as screening tools and may improve prediction, risk or early diagnosis of Alzheimer’s disease. "We are on the cusp of a new generation of therapies for Alzheimer's disease, but the important role of diagnostics has been missing from the conversation. Patients today are typically screened for Alzheimer's disease only after signs of cognitive impairment emerge and often by expensive methods, such as brain imaging and cerebrospinal fluid taps, which only specialists can perform. As new, efficacious therapies come to the forefront, the need for scalable, less invasive, and more cost-effective diagnostics, including in primary care settings, will grow," said Michael K. Racke, M.D., Neurology Medical Director, Quest Diagnostics. The National Institute on Aging and the Alzheimer’s Association (NIA-AA) recognize the value of amyloid PET and other imaging procedures along with CSF Aβ as AD biomarkers, and currently amyloid PET is the only FDA-approved diagnostic for detection of brain amyloid. However, these methods remain underutilized, perhaps because of cost, unavailability in rural areas, complexity, and lack of perceived usefulness by clinicians. Many believe PET is unlikely to be widely implemented in clinical practice due to logistics and access challenges, while lumbar puncture is considered complicated, time-consuming, and invasive. Plasma-based biomarkers offer practical advantages over imaging and CSF measurements, making plasma tests more accessible to clinicians and patients.
Plasma amyloid Beta
Plasma Aβ has been reported as a potential predictor of AD; however, results are inconsistent according to research findings from several studies. Aβ42 is highly labile and prone to aggregate, making its concentrations susceptible to variation in pre-analytical processing. The ratio of Aβ42/Aβ40 in plasma may be more useful than Aβ peptides individually, and it appears to be associated with an increased risk of progression to AD dementia and more significant cognitive decline. Correlation or partial correlation between Aβ42/Aβ40 plasma ratio and amyloid PET and CSF has been reported. Multiple researchers described a decrease in plasma Aβ42/Aβ40 ratio measured by an immunoprecipitation and liquid chromatography-mass spectrometry assay (IP-MS), providing evidence that plasma Aβ42/Aβ40 can accurately diagnose brain amyloidosis (AUC = 0.88). This result supports the use of Aβ42/Aβ40 ratio in plasma as a screening tool for those at risk of AD dementia. Lower plasma Aβ42/40 ratio has been associated with a twofold increased risk of clinical progression to MCI or dementia. Additionally, it was found that individuals with positive plasma Aβ42/Aβ40 but negative amyloid PET scan have a 15-fold higher risk of converting to amyloid PET-positive (p = 0.01), which suggests that Aβ42/Aβ40 ratio becomes positive earlier than the established amyloid PET threshold used in this study. Plasma biomarkers currently employed in clinical practice.
This blood test measures the Aβ42/40 ratio in the blood. It was launched onto the market in May 2022, at a price of $500. It can be ordered directly by your physician. Unlike the other tests, this diagnostic is covered by certain in-network health providers for many insurance health plans.
AD-Detect measures the amyloid beta ratio of two blood-based biomarkers, beta amyloid 42 (Aβ42) and beta amyloid (Aβ40), for early detection of AD in blood plasma using liquid chromatography–tandem mass spectrometry (LC–MS/MS). This analysis-based assay provides a more straightforward option than positron emission tomography (PET) imaging and a less invasive procedure than cerebrospinal fluid (CSF) sampling. If the ratio is low, this indicates an increased risk of developing Alzheimer’s disease. 2. PrecivityAD™ blood test by C₂N Diagnostics
C₂N’s lab measures the concentrations of amyloid 42 and 40 and checks for the presence of apolipoprotein E. From these measures, C₂N’s automated method generates an Amyloid Probability Score to suggest the likelihood of amyloid plaques in the brain. A high score is consistent with a high level of amyloid plaques found on a PET scan. Studies have demonstrated that a lower plasma Aβ42/Aβ40 ratio, in combination with the well-established risk factors of APOE4 status and age, correlate with brain amyloidosis as measured using amyloid PET imaging.
The test is not covered by insurance or Medicare, but at $1,250 the cost is slightly lower than imaging tests like PET scans. C₂N Diagnostics, the company behind the test, also offers a financial assistance program to patients based on income. The company recently introduced the p-tau multi-analyte assay for research use only. Shown at this year’s Alzheimer's Association International Conference, combining C2N’s plasma Aβ42/40 ratio assay with p-tau217 ratio assay yields a blood test with comparable accuracy as the current gold standards for Alzheimer’s brain pathology detection (amyloid PET imaging or cerebrospinal fluid tests), albeit at a lower cost, with less invasive sampling needs, and much lower complexity. 3. Simoa p-Tau181 test by Quanterix This blood test was publicly launched at the end of July 2022, providing a measurement of the levels of a protein called p-tau181 in the blood. Higher levels of this protein are linked to beta-amyloid pathology. But a 2022 study found that p-tau181 may be inaccurate as a diagnostic biomarker for Black people. Phosphorylated tau at threonine 181 (p-tau181) was elevated in a group of people with amyloid-positive mild cognitive impairment or Alzheimer's dementia, compared with all other groups. Researchers have shown that the plasma biomarker p-tau231 is particularly suitable for capturing incipient brain changes related to the amyloid protein, before the plaque of this protein manifests itself. This was published in Nature Medicine, and indicate that p-tau231 is a promising blood biomarker for detecting cognitively healthy individuals at high risk of developing Alzheimer's disease. This finding will help drive clinical trials on the preclinical phase of Alzheimer's disease. 4. Glial fibrillar acid protein (GFAP) was elevated in the Alzheimer's group and in Lewy body dementia. Plasma glial fibrillary acidic protein (GFAP) is a marker of astroglial activation and astrocytosis. This test is still in investigation and not yet ready for clinical use at this time, but you can learn more about it at: https://neogenomics.com/test-menu/gfap 5. Neurofilament light (NfL) was elevated in all people with dementias compared with controls. Low levels of NfPs are constantly released from neurons into the extracellular space and ultimately reach the cerebrospinal fluid (CSF) and blood under physiological conditions throughout normal brain development, maturation, and aging. NfP levels in CSF and blood rise above normal in response to neuronal injury and neurodegeneration independent of the cause. In AD, abnormal aggregation and alterations of neurofilaments have been reported with increased levels of plasma and the CSF NfL subunit proportional to axonal damage. NfL can be measured with immunoassays in CSF and plasma and studies show it may serve as a promising diagnostic and prognostic neurodegeneration marker for AD. High plasma NfL levels appear to correlate with poor cognition and brain atrophy, and distinguish between AD, MCI, and healthy controls in sporadic AD, with higher values among subjects with MCI associated with more rapid brain atrophy. A recent study on an ADNI cohort found a correlation between plasma and CSF NfLsuggesting that blood measurements reflect brain pathophysiology. In summary
Given what we know of the many shared mechanisms of injury between SARS CoV 2, spike protein and other viral fragments, clinicians will be overwhelmed with “memory loss” evaluations. These biomarkers have arrived on the scene just in time to provide more insight for the benefit of our patients and ideally for improved outcomes.
In health and healing, Dr. Suzanne Gazda References: Hardy, J., Higgins, G. Alzheimer's Disease: The Amyloid Cascade Hypothesis. Science. (1992) https://www.science.org/doi/abs/10.1126/science.1566067 Morris, G.P., Clark, I.A. & Vissel, B. Inconsistencies and Controversies Surrounding the Amyloid Hypothesis of Alzheimer's Disease. acta neuropathol commun 2, 135 (2014). https://doi.org/10.1186/s40478-014-0135-5 Kater, M., Huffels, C. et al. Prevention of microgliosis halts early memory loss in a mouse model of Alzheimer’s disease. Brain, Behavior, and Immunity. (2023) https://doi.org/10.1016/j.bbi.2022.10.009 Oluwaseyi Olayinka, Olaniyi O. Olayinka et al. Toxic Environmental Risk Factors for Alzheimer’s Disease: A Systematic Review. Aging Medicine and Healthcare. (2017) https://www.agingmedhealthc.com/wp-content/uploads/2019/03/AMH-2019v10i1_2_jcgg-2017-0027.pdf Hardy-Sosa A, León-Arcia K, Llibre-Guerra JJ, Berlanga-Acosta J, Baez SC, Guillen-Nieto G and Valdes-Sosa PA (2022) Diagnostic Accuracy of Blood-Based Biomarker Panels: A Systematic Review. Front. Aging Neurosci. 14:683689. doi: 10.3389/fnagi.2022.683689 West T, Kirmess KM, Meyer MR, et al. A blood-based diagnostic test incorporating plasma Aβ42/40 ratio, ApoE proteotype, and age accurately identifies brain amyloid status: findings from a multi cohort validity analysis. Mol Neurodegener. 2021;16(1):30. doi:10.1186/s13024-021-00451-6 Kirmess, K., Meyer, M. et al. The PrecivityAD™ test: Accurate and reliable LC-MS/MS assays for quantifying plasma amyloid beta 40 and 42 and apolipoprotein E proteotype for the assessment of brain amyloidosis. Clinica Chimica Acta. 2021. https://doi.org/10.1016/j.cca.2021.05.011 Milà-Alomà, M., Ashton, N.J., Shekari, M. et al. Plasma p-tau231 and p-tau217 as state markers of amyloid-β pathology in preclinical Alzheimer’s disease. Nat Med 28, 1797–1801 (2022). https://doi.org/10.1038/s41591-022-01925-w Park JE, Gunasekaran TI, Cho YH, Choi S-M, Song M-K, Cho SH, Kim J, Song H-C, Choi KY, Lee JJ, Park Z-Y, Song WK, Jeong H-S, Lee KH, Lee JS, Kim BC. Diagnostic Blood Biomarkers in Alzheimer’s Disease. Biomedicines. 2022; 10(1):169. https://www.mdpi.com/2227-9059/10/1/169 Hansson, O, Edelmayer, RM, Boxer, AL, et al. The Alzheimer's Association appropriate use recommendations for blood biomarkers in Alzheimer's disease. Alzheimer's Dement. 2022; 1- 18. https://doi.org/10.1002/alz.12756. Pontecorvo MJ, Lu M, Burnham SC, et al. Association of Donanemab Treatment With Exploratory Plasma Biomarkers in Early Symptomatic Alzheimer Disease: A Secondary Analysis of the TRAILBLAZER-ALZ Randomized Clinical Trial. JAMA Neurol. Published online October 17, 2022. doi:10.1001/jamaneurol.2022.3392
0 Comments
Your comment will be posted after it is approved.
Leave a Reply. |
AuthorSuzanne Gazda M.D. Neurologist Archives
January 2024
Categories |


 RSS Feed
RSS Feed