|
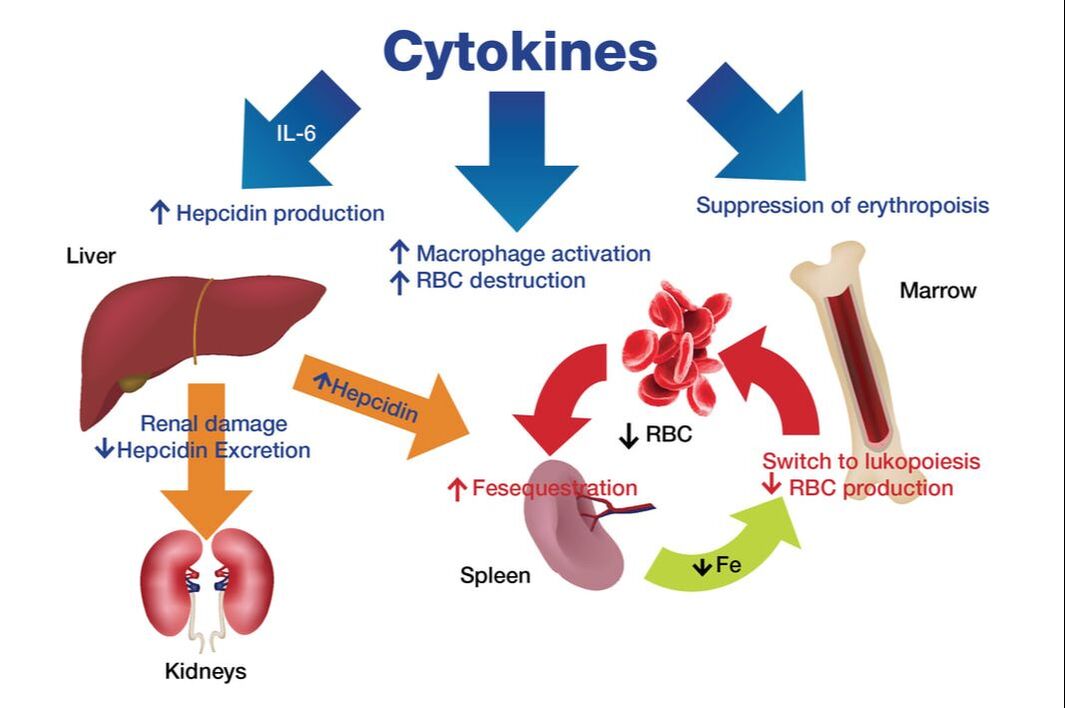
Pediatric acute-onset neuropsychiatric syndrome (PANS) is a complex neurological disorder that more scientists are studying in order to learn more about the origins of this illness that along with PANDAS (pediatric autoimmune neuropsychiatric disorders associate with streptococcal infections) is estimated to affect 1 in 200 children. While PANDAS is thought to occur following an infection, the diagnosis for PANS is a clinical one based on the original working criteria first introduced by Dr. Susan Swedo.* A recent preprint study, “Hypoferritinemia and iron deficiency in youth with pediatric acute onset neuropsychiatric syndrome" led by Jennifer Frankovich, MD from the Stanford PANS Clinic explored the potential association between hypoferritinemia (low iron storage in the ferritin form) and the development over time of neuropsychiatric symptoms. The authors concluded that in patients with PANS and iron deficiency, “clinicians should consider the possibility of inflammation as the cause especially if iron deficiency cannot be explained by diet and blood loss.” 1 Additional findings released by the investigating team were as follows: ▪ Children with anxiety, depression, tic disorders, attention-deficit/hyperactivity disorder, febrile seizures, breath holding spells, and fibromyalgia, are more likely to be iron deficient. ▪ 1 in 4 PANS patients (27%) had hypoferritinemia. ▪ 75% of those cases occurred during a PANS “flare” (abrupt worsening of neuropsychiatric symptoms. ▪ Patients with hypoferritinemia had worse global impairment, more comorbid inflammatory diseases, and exhibited a chronic course of PANS illness.” ▪ An estimated 3-8% of the PANS patients had iron deficiency. That rate is nearly 2 times higher than healthy subjects. ▪ Out of the patients who had an iron workup, 14% had an iron deficiency. And about half of those patients also had anemia. ▪ The hypoferritinemic group of patients had a higher rate of chronic PANS illness (69% vs. 46%) and higher global impairment scores. (When ferritin levels were measured.) ▪ Comorbid inflammatory diseases were also more commonly seen in the hypoferritinemic group. ▪ Ferritin levels returned to normal for the majority (69%) of the hypoferritinemic patients after they received treatment. ▪ Therapies included IVIG, methylprednisolone, prednisone bursts, NSAID or a combination of these treatments. Ferritin and its significance. Free iron, not typically found in large amounts in the bloodstreams, can be toxic as it can act as a catalyst to the formation of free radicals that subsequently damage cells. Ordinarily, our systems draw upon several protective mechanisms to bind iron in various tissue compartments. This way, the iron is stored complexed to protein as ferritin or hemosiderin. Ferritin serum level correlates with the total values of iron stores in organism stores. Low serum ferritin (less than 15 ng/ml for general population with some labs using age and gender-specific norms) is highly specific for iron deficiency. But we have long known about something called anemia of inflammation (AI), formerly also called anemia of chronic disease or anemia of chronic disorders. This disorder is usually a mild to moderately severe anemia (hemoglobin rarely lower than 8 g/d) that develops in the setting of infection, inflammatory disease or malignancy.2 When the protective mechanisms go awry. In the study linking hypoferritinanemia and PANS, the pathophysiology involved here includes several systemic actions that result in this low ferritin storage: ▪ Mildly shortened erythrocyte survival (increased destruction) due to macrophage activation by inflammatory cytokines. ▪ Hypoferremia, iron-restricted erythropoiesis from cytokine-stimulated hepcidin increase. The hormone hepcidin, a peptide, is the principal regulator of iron absorption and its distribution to tissues, hepcidin acts by regulating the iron delivery to plasma from macrophages that recycle senescent erythrocytes, from duodenal enterocytes that absorb dietary iron and from hepatocytes involved in iron storage.
▪ Inflammation will increase plasma concentrations of hepcidin; suppression of erythropoiesis by direct effects of cytokines on the marrow Variable effects of inflammation on erythropoietin production, renal excretion of hepcidin. What treatment approach can be pursued? It is best to identify and treat the root cause of the underlying disease and the contributors to chronic inflammation; in some cases there may be an underlying infectious trigger as well (e.g. streptococcus, varicella etc.) and these should be treated accordingly as well. The significance of this study and corresponding information. Iron is of central importance to many vital processes because the metal is a catalytic component of crucial metabolic enzymes in the citric acid cycle, mitochondrial respiration, replication, myelin production and/or neurotransmitter synthesis (dopamine and serotonin). We know that in the pathophysiology of a disorder such as idiopathic restless legs syndrome, for example, disturbances of dopaminergic function and alterations of iron homeostasis are involved in its pathogenesis. We also know the impact in PANS and PANDAS of dysregulated dopamine in the basal ganglia. Children with iron deficiency conditions are prone to developmental delays, reduced school performance, and behavioral disorders. An association has been found between low Hb and smaller brain volume, providing a potential mechanism to explain the greater risk of adults with dementia in individuals with anemia. The overall negative effect of low Hb on brain outcomes in these data supports the notion that chronic hypoxia accelerates cognitive decline and increases the risk of brain disease.3 Certainly these and other available studies shed more light on PANS and the role of inflammation in the course of this disease. But, as noted by the principal investigators, further research must be conducted to more fully explore the foundations of these neuropsychiatric disorders. Toward this goal, integrative medicine, which recognizes that all systems and processes are intricately connected, can play an important role in identifying expanded treatment protocols…and ultimately giving more patients and families alike the hope of improved health outcomes. In health, Dr. Suzanne Gazda References: *The PANDAS Network http://pandasnetwork.org/medical-information/ 1 Chan A, Karpel H, Spartz E, et al. Hypoferritinemia and iron deficiency in youth with pediatric acute-onset neuropsychiatric syndrome [published online ahead of print, 2020 Aug 3]. Pediatr Res. 2020;10.1038/s41390-020-1103-3. doi:10.1038/s41390-020-1103-3 https://pubmed.ncbi.nlm.nih.gov/32746449/ 2 Nemeth, E., & Ganz, T. (2014). Anemia of inflammation. Hematology/oncology clinics of North America, 28(4), 671–vi. https://doi.org/10.1016/j.hoc.2014.04.005 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4115203/ 3 Jonassaint, C. R., Varma, V. R., Chuang, Y. F., Harris, G. C., Yasar, S., Polinder-Bos, H., & Carlson, M. C. (2014). Lower hemoglobin is associated with poorer cognitive performance and smaller brain volume in older adults. Journal of the American Geriatrics Society, 62(5), 972–973. https://doi.org/10.1111/jgs.12810 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4292910/ Additional reading: IntechOpen https://www.intechopen.com/books/iron-deficiency-anemia/neurocognitive-dysfunctions-in-iron-deficiency-patients The Atlantic https://www.theatlantic.com/health/archive/2013/01/the-iron-in-our-blood-that-keeps-and-kills-us/266936/
1 Comment
Tonya
1/5/2022 08:45:42 pm
Thank you for the article.
Reply
Your comment will be posted after it is approved.
Leave a Reply. |
Authorby Suzanne Gazda M.D. Archives
August 2021
Categories |
 RSS Feed
RSS Feed