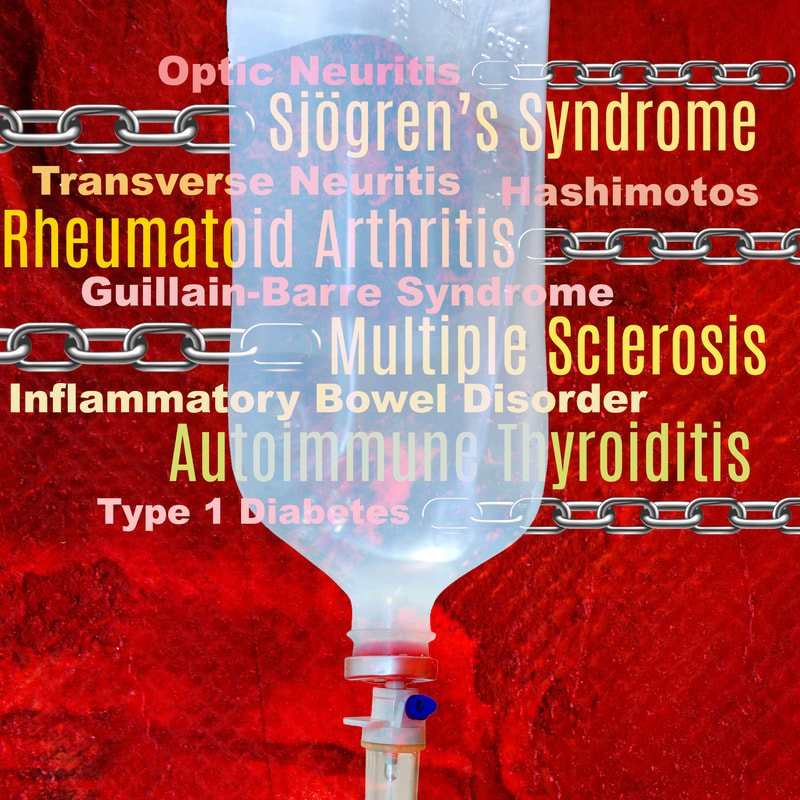
About 25% of patients with autoimmune disease will have more than one autoimmune disorder; patients with rheumatoid arthritis (RA), multiple sclerosis (MS), autoimmune thyroiditis, Sjögren’s syndrome or similar conditions carry a markedly increased risk of having another autoimmune disease.
For example, the estimated prevalence of MS in the general population is 0.1% but the prevalence of the disease jumps to 0.5% in patients with inflammatory bowel disorder, suggesting a 1.5 to 5-fold increase in the risk of developing MS in patients with IBD.1 There is also a link between RA and IBD, RA, MS, and Hashimotos, Sjogrens and Type 1 diabetes – really, it’s quite extraordinary when you realize there are so many links between just as many of these serious conditions. These relationships clearly present as a case of a mistaken and activated immune system and, if the immune system already follows the same autoimmune pattern, there is subsequently a higher possibility as well as probability that there will be another target. There have been reports as well of central nervous system (CNS) demyelination that suggests a triggering or exacerbating effect in demyelination diseases such as MS that may very well be induced by treatment with anti-TNF-α.2 TNF-α (tumor necrosis factor alpha) is a well-known pro-inflammatory and immunoregulatory cytokine. Elevated levels of this cytokine are locally and systemically in patients with inflammatory disorders like arthritis. Drugs that block the effect of TNF-α modulate the activity of many autoimmune diseases. Depending on their mechanism of action, anti-TNF-α drugs are divided into monoclonal antibodies (Infliximab, Adalimumab, Golimumab, and Certolizumab) and soluble TNF-α receptors (Etanercept).3 I feel as if we go around and around with the concept of an increased risk of shared autoimmune disease in our patient populations. But we must be aware that there is an increased risk of MS developing in those patients who are on TNF-α inhibitors that are sometimes used to treat other autoimmune problems such as RA. Other examples report an increased risk of MS, optic neuritis, Guillain-Barre syndrome, and transverse neuritis developing in patients on TNF alpha inhibitors.4 So I feel that physicians must be cautious when prescribing anti-TNF-α in patients with a family history of MS and also to reconsider the risk of MS in patients being treated for autoimmune diseases. And still more… One published report additionally linked the use of rituximab for treatment of lymphoma with the initiation of IBD in certain elderly patients. Authors noted that "The association of rituximab and ileocolitis suggests a protective effect of CD20 + lymphocytes in the gut, and implicates their depletion to the development and exacerbation of inflammatory bowel disease." There have also been additional instances of other disease initiation including rituximab-induced psoriasis, finding that "B cell-depleted environment may induce abnormal T cell responses, possibly provoked either by subclinical infection or by the removal of mechanisms whereby B cells regulate T cells."5 We should expect this to be a possible side effect with any of the B cell depleting therapies (Ocrevus and Kimsimpta). So, if your patient is on a B cell blocking drug, this medication will likely need to be stopped or it may be hard to control the Crohn's or other resulting condition. No doubt the advancement of biologics therapies has been profoundly helpful in the treatment of various autoimmune diseases. But sometimes our immune modulating therapies can come with unexpected penalties. Could IVIG be a therapeutic alternative? Many patients with Crohn's disease are on immunosuppressive therapies. However, there have been case reports of progressive multifocal leukoencephalopathy (PML), an infection that damages the material (myelin) that covers and protects nerves in the white matter of the brain, in these patient populations. Therefore, it stands to reason we must consider in some instances alternative treatments for these conditions to potentially decrease the risk of developing additional disorders. Intravenous immunoglobulin (IVIG) has been used for years in Crohn’s and could be a good choice in some patients, especially those who have more than one autoimmune disease, in order to target therapies in the safest way possible. Published findings noted that “A review of the evidence identified indicates that IVIG can induce a rapid and significant improvement in aminosalicylate- and steroid-resistant CD, often within days of the initial administration."6 Immunoglobulin has been employed as a safe and effective protocol in many autoimmune disorders, but without the same degree of treatment “fallout.” We’ve written about the use of IVIG therapies not only for neurological diseases, but as we are now seeing, it also has applicable benefits in treating COVID 19. IVIG does not target the virus, but is used to help calm the overactive immune response seen in the disease by decreasing the instance of multiple cytokines at one time.7 In its use for treatment of autoimmune disease, IVIG has a multimodal method of action (MOA). A number of studies have shown the benefits of IVIg and its anti-inflammatory effects as well as an ability to regulate immune balance. IVIg additionally has the capacity to eliminate clinical autoimmunity, restore a state of tolerance, and reinstate physiologic homeostasis.8 There is still much to be learned and better understood about how conventional treatments for neuroimmune and autoimmune conditions may impact disease initiation in some patient groups. But we do know that IVIG has been extremely well tolerated and yielded excellent results as part of a comprehensive program. It’s well advised to consider this in any patient who presents with a preexisting condition if there are concerns about employing medications. Communication is also critical, between any clinicians participating in patient care, as well with the patient themselves and any supporting family members. Together, we can arrive at a more successful treatment plan unique to the individual and their health needs…and that is the basic tenet and very real benefit of integrative medicine. In health and hope, Dr. Suzanne Gazda References: 1 Dziadkowiec K N, Stawinski P, Radadiya D, et al. (July 30, 2020) Is Multiple Sclerosis an Extra-Intestinal Manifestation of Inflammatory Bowel Disease? Food for Thought. Cureus 12(7): e9485. doi:10.7759/cureus.9485 2 Kemanetzoglou, E., & Andreadou, E. (2017). CNS Demyelination with TNF-α Blockers. Current neurology and neuroscience reports, 17(4), 36. https://doi.org/10.1007/s11910-017-0742-1 3 Van Oosten BW, Barkhof F, Truyen L, et al. Increased MRI activity and immune activation in two multiple sclerosis patients treated with monoclonal anti-tumor necrosis factor antibody cA2. Neurology. 1996;47(6):1531–1534) 4 Alnasser Alsukhni, R., Jriekh, Z., & Aboras, Y. (2016). Adalimumab Induced or Provoked MS in Patient with Autoimmune Uveitis: A Case Report and Review of the Literature. Case reports in medicine, 2016, 1423131. https://doi.org/10.1155/2016/1423131 5 Varma P, Falconer J, Aga A, Prince HM, Pianko S. Rituximab-induced Crohn's disease. Scand J Gastroenterol. 2017 May;52(5):606-608. doi: 10.1080/00365521.2017.1280530. Epub 2017 Jan 27. PMID: 28129697. 6 Moshe Rogosnitzky, Rachel Danks, Daniel Hol. Intravenous immunoglobulin for the treatment of Crohn's disease. Autoimmunity Reviews (2012).Volume 12, Issue 2. Pages 275-280. ISSN 1568-9972. https://doi.org/10.1016/j.autrev.2012.04.006. https://www.sciencedirect.com/science/article/pii/S1568997212000961 7 Suzanne Gazda MD (www.suzannegazdamd.com) https://www.suzannegazdamd.com/blog/with-more-potential-uses-for-more-diseases-ivig-gains-enhanced-awareness-for-its-multimodal-properties 8 Suzanne Gazda MD (www.suzannegazdamd.com) https://www.suzannegazdamd.com/blog/ivig-in-autoimmune-disease-therapies
1 Comment
Your comment will be posted after it is approved.
Leave a Reply. |
Authorby Suzanne Gazda M.D. Archives
August 2021
Categories |
 RSS Feed
RSS Feed